当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peroxo-Diiron(III/III) as the Reactive Intermediate for N-Hydroxylation Reactions in the Multidomain Metalloenzyme SznF: Evidence from Molecular Dynamics and Quantum Mechanical/Molecular Mechanical Calculations
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-13 , DOI: 10.1021/acscatal.3c00174 Jia Liu 1 , Zikuan Wang 2 , Xianhang Sang 1 , Xuan Zhang 3 , Binju Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-13 , DOI: 10.1021/acscatal.3c00174 Jia Liu 1 , Zikuan Wang 2 , Xianhang Sang 1 , Xuan Zhang 3 , Binju Wang 1
Affiliation
|
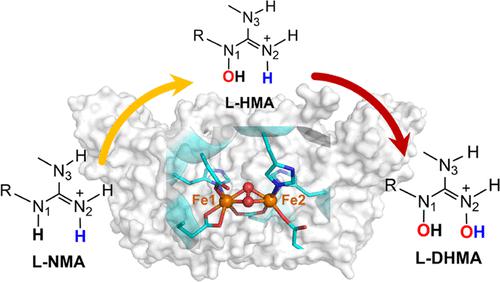
Upon oxygen activation, the non-heme diiron enzymes can generate various active species for oxidative transformations. In this work, the catalytic mechanism of the diiron active site (heme-oxygenase-like diiron oxidase (HDO) domain) in SznF has been comprehensively studied by molecular docking, classical molecular dynamics (MD) and quantum mechanical/molecular mechanical (QM/MM) MD simulations, and hybrid QM/MM calculations. The HDO domain of SznF catalyzes the selective hydroxylation of Nω-methyl-l-arginine (l-NMA) to generate Nδ-hydroxy-Nω-methyl-l-Arg (l-HMA) and Nδ,Nω-dihydroxy-Nω,-methyl-l-Arg (l-DHMA), which is a key step in the synthesis of the nitrosourea pharmacophore of the pancreatic cancer drug streptozotocin (SZN). Our study shows that the peroxo-diiron(III/III) intermediate in Sznf maintains a butterfly-like conformation, while the further protonation of the diiron(III/III) intermediate is found to be inaccessible and unfavorable thermodynamically. Among various mechanisms, we found that the most favorable mechanism involves the nucleophilic attack of the guanidium group onto the peroxo group of P1, which drives the heterolytic cleavage of the O–O bond. Moreover, the selectivity of N-hydroxylation by the peroxo-diiron(III/III) intermediate can be fully supported by MD simulations, suggesting that the peroxo-diiron(III/III) is the reactive intermediate for N-hydroxylation in SznF. The present study expands our understanding on the O2 activation and N-hydroxylation by the non-heme diiron enzymes.
中文翻译:

过氧二铁 (III/III) 作为多域金属酶 SznF 中 N-羟基化反应的反应中间体:来自分子动力学和量子力学/分子力学计算的证据
在氧活化后,非血红素二铁酶可以产生各种用于氧化转化的活性物质。在这项工作中,通过分子对接、经典分子动力学(MD)和量子力学/分子力学(QM/ MM) MD 模拟和混合 QM/MM 计算。SznF的HDO结构域催化N ω -甲基- l -精氨酸(l -NMA)选择性羟基化生成N δ -羟基-N ω -甲基- l -Arg(l -HMA)和N δ ,N ω -二羟基-N ω,-甲基- l -Arg ( l-DHMA),这是胰腺癌药物链脲佐菌素 (SZN) 的亚硝基脲药效团合成的关键步骤。我们的研究表明,Sznf 中的过氧二铁 (III/III) 中间体保持蝴蝶状构象,而发现二铁 (III/III) 中间体的进一步质子化在热力学上是不可接近和不利的。在各种机制中,我们发现最有利的机制涉及胍基对 P1 的过氧基的亲核攻击,从而驱动 O-O 键的异裂解。此外,MD 模拟完全支持过氧二铁 (III/III) 中间体对 N-羟基化的选择性,表明过氧二铁 (III/III) 是 SznF 中 N-羟基化的反应中间体。2非血红素二铁酶的激活和 N-羟基化。
更新日期:2023-04-13
中文翻译:

过氧二铁 (III/III) 作为多域金属酶 SznF 中 N-羟基化反应的反应中间体:来自分子动力学和量子力学/分子力学计算的证据
在氧活化后,非血红素二铁酶可以产生各种用于氧化转化的活性物质。在这项工作中,通过分子对接、经典分子动力学(MD)和量子力学/分子力学(QM/ MM) MD 模拟和混合 QM/MM 计算。SznF的HDO结构域催化N ω -甲基- l -精氨酸(l -NMA)选择性羟基化生成N δ -羟基-N ω -甲基- l -Arg(l -HMA)和N δ ,N ω -二羟基-N ω,-甲基- l -Arg ( l-DHMA),这是胰腺癌药物链脲佐菌素 (SZN) 的亚硝基脲药效团合成的关键步骤。我们的研究表明,Sznf 中的过氧二铁 (III/III) 中间体保持蝴蝶状构象,而发现二铁 (III/III) 中间体的进一步质子化在热力学上是不可接近和不利的。在各种机制中,我们发现最有利的机制涉及胍基对 P1 的过氧基的亲核攻击,从而驱动 O-O 键的异裂解。此外,MD 模拟完全支持过氧二铁 (III/III) 中间体对 N-羟基化的选择性,表明过氧二铁 (III/III) 是 SznF 中 N-羟基化的反应中间体。2非血红素二铁酶的激活和 N-羟基化。















































 京公网安备 11010802027423号
京公网安备 11010802027423号