当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioorthogonal Peptide Macrocyclization Using Oxime Ligation
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-13 , DOI: 10.1021/acs.orglett.3c00695
Lani J Davies 1 , Laura M Shuttleworth 1 , Xiaobai Zhang 1 , Sherry Peng 1 , Christoph Nitsche 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-13 , DOI: 10.1021/acs.orglett.3c00695
Lani J Davies 1 , Laura M Shuttleworth 1 , Xiaobai Zhang 1 , Sherry Peng 1 , Christoph Nitsche 1
Affiliation
|
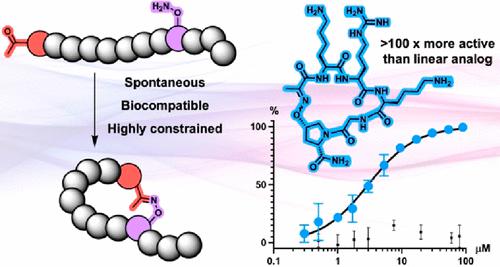
The biocompatible synthesis of constrained peptides is challenging. Oxime ligation is a bioorthogonal technique frequently used for protein bioconjugation. We report a straightforward method to install N-terminal ketones and aminooxy side chains during standard solid-phase peptide synthesis. Cyclization occurs spontaneously after acidic cleavage or in aqueous buffer. We demonstrate the facile synthesis of protease inhibitors with varying conformational constraint. The most constrained peptide displayed an activity 2 orders of magnitude higher than its linear analog.
中文翻译:

使用肟连接的生物正交肽大环化
受限肽的生物相容性合成具有挑战性。肟连接是一种经常用于蛋白质生物偶联的生物正交技术。我们报告了一种在标准固相肽合成过程中安装 N 末端酮和氨基氧基侧链的简单方法。环化在酸性裂解后或在水性缓冲液中自发发生。我们展示了具有不同构象约束的蛋白酶抑制剂的简便合成。最受限制的肽显示出比其线性类似物高 2 个数量级的活性。
更新日期:2023-04-13
中文翻译:

使用肟连接的生物正交肽大环化
受限肽的生物相容性合成具有挑战性。肟连接是一种经常用于蛋白质生物偶联的生物正交技术。我们报告了一种在标准固相肽合成过程中安装 N 末端酮和氨基氧基侧链的简单方法。环化在酸性裂解后或在水性缓冲液中自发发生。我们展示了具有不同构象约束的蛋白酶抑制剂的简便合成。最受限制的肽显示出比其线性类似物高 2 个数量级的活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号