Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-04-13 , DOI: 10.1016/j.bioorg.2023.106538 Shams Aaghaz 1 , Chander S Digwal 2 , Naziya Neshat 3 , Indresh K Maurya 4 , Vinod Kumar 5 , Kulbhushan Tikoo 5 , Rahul Jain 1 , Ahmed Kamal 6
|
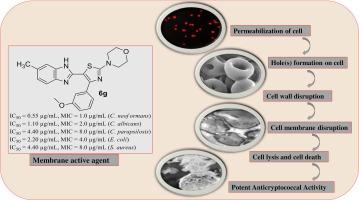
In spite of several attempts to develop newer pharmacophores as potential antimicrobial agents, the benzimidazole scaffold is still considered as one of the most sought after structural component towards the design of compounds that act against a wide spectrum of microbes. Herein, we report the design and synthesis of a new structural class of 4-(1,3-thiazol-2-yl)morpholine-benzimidazole hybrids as antimicrobial agents. The most potent analog, 6g shows IC50 of 1.3 µM, 2.7 µM, 10.8 µM, 5.4 µM and 10.8 µM against Cryptococcus neoformans, Candida albicans, Candida parapsilosis, Escherichia coli and Staphylococcus aureus, respectively. Interestingly 6g exhibits selectivity towards the cryptococcal cells with fungicidal behavior. Propidium iodide uptake study shows permeabilization of pathogenic cells in the presence of 6g. Flow cytometric analysis confirms that cell death is predominantly due to apoptosis. Moreover, electron microscopic analysis specifies that it shrinks, disrupts and initiate pore(s) formation in the cell membrane leading to cell lysis.
中文翻译:

4-(1,3-噻唑-2-基)吗啉-苯并咪唑杂化物作为一类新结构的抗菌剂的合成、生物学评价和机理研究
尽管多次尝试开发更新的药效团作为潜在的抗菌剂,但苯并咪唑支架仍然被认为是设计对广谱微生物起作用的化合物时最受追捧的结构组分之一。在此,我们报告了一种新结构类 4-(1,3-thiazol-2-yl)moroline-benzimidazole 杂化物作为抗菌剂的设计和合成。最有效的类似物6g显示 IC 50分别为 1.3 µM、2.7 µM、10.8 µM、5.4 µM 和 10.8 µM 对抗新型隐球菌、白色念珠菌、近光滑念珠菌、大肠杆菌和金黄色葡萄球菌。有趣的是6g对具有杀真菌行为的隐球菌细胞表现出选择性。碘化丙锭摄取研究显示在6g存在下致病细胞的透化作用。流式细胞术分析证实细胞死亡主要是由于细胞凋亡。此外,电子显微镜分析表明它会收缩、破坏并引发细胞膜中的孔隙形成,从而导致细胞裂解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号