当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complementary asymmetric routes to fused tricyclic (R)-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinolines and (R)-1,2,3,4,5,5a,6,7-octahydro-[1,4]diazepino[1,2-a]quinolines
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-09-24 19:14:45 Thomas O. Schrader, Michelle Kasem, Qi Sun, Chunrui Wu, Albert Ren, Graeme Semple
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-09-24 19:14:45 Thomas O. Schrader, Michelle Kasem, Qi Sun, Chunrui Wu, Albert Ren, Graeme Semple
|
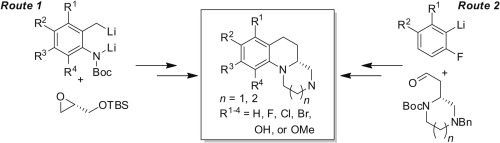
Two distinct enantioselective approaches to (R)-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinolines and (R)-1,2,3,4,5,5a,6,7-octahydro-[1,4]diazepino[1,2-a]quinolines, low MW tricyclic organic scaffolds with a high degree of molecular complexity, are described. The key transformation in route 1 is the lateral lithiation of an N-Boc-o-toluidine and dianion trap with (S)-tert-butyldimethyl(oxiran-2-ylmethoxy)silane. An intramolecular SN2 cyclization then forms the optically pure tetrahydroquinoline core. Route 2 involves the coupling of (R)-2-(4-benzyl-1-(Boc)piperazin-2-yl)acetaldehyde or (R)-2-(4-benzyl-1-(Boc)-1,4-diazepan-2-yl)acetaldehyde with an aryllithium and a subsequent intramolecular SNAr reaction to form the tricycle. Both synthetic routes were valuable for preparing and identifying ligands targeting GPCRs expressed in the central nervous system (CNS).
中文翻译:

稠合三环(R)-2,3,4,4a,5,6-六氢-1H-吡嗪并[1,2-a]喹啉和(R)-1,2,3,4,5的互补不对称路线5a,6,7-八氢-[1,4]二氮杂[1,2-a]喹啉
(R)-2,3,4,4a,5,6-hexahydro-1H-pyrazino [1,2-a]喹啉和(R)-1,2,3,4,5,5a的两种不同的对映选择性方法描述了6,7-八氢-[1,4]二氮杂[1,2-a]喹啉,低分子量的三环有机支架,具有高度的分子复杂性。路线1中的关键转变是N-Boc-邻甲苯胺和二价离子阱与(S)-叔丁基二甲基(环氧乙烷-2-基甲氧基)硅烷的侧向锂化反应。然后分子内的S N 2环化形成光学纯的四氢喹啉核心。路线2涉及(R)-2-(4-苄基-1-(Boc)哌嗪-2-基)乙醛或(R)-2-(4-苄基-1-(Boc)-1,4)的偶联-二氮杂-2-基)乙醛与芳基锂和随后的分子内S NAr反应形成三环。两种合成途径对于制备和鉴定靶向中枢神经系统(CNS)中表达的GPCR的配体都是有价值的。
更新日期:2016-09-25
中文翻译:

稠合三环(R)-2,3,4,4a,5,6-六氢-1H-吡嗪并[1,2-a]喹啉和(R)-1,2,3,4,5的互补不对称路线5a,6,7-八氢-[1,4]二氮杂[1,2-a]喹啉
(R)-2,3,4,4a,5,6-hexahydro-1H-pyrazino [1,2-a]喹啉和(R)-1,2,3,4,5,5a的两种不同的对映选择性方法描述了6,7-八氢-[1,4]二氮杂[1,2-a]喹啉,低分子量的三环有机支架,具有高度的分子复杂性。路线1中的关键转变是N-Boc-邻甲苯胺和二价离子阱与(S)-叔丁基二甲基(环氧乙烷-2-基甲氧基)硅烷的侧向锂化反应。然后分子内的S N 2环化形成光学纯的四氢喹啉核心。路线2涉及(R)-2-(4-苄基-1-(Boc)哌嗪-2-基)乙醛或(R)-2-(4-苄基-1-(Boc)-1,4)的偶联-二氮杂-2-基)乙醛与芳基锂和随后的分子内S NAr反应形成三环。两种合成途径对于制备和鉴定靶向中枢神经系统(CNS)中表达的GPCR的配体都是有价值的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号