当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidase Heterotetramer Completes 1-Azabicyclo[3.1.0]hexane Formation with the Association of a Nonribosomal Peptide Synthetase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-12 , DOI: 10.1021/jacs.2c12507 Yiyuan Cheng 1 , Xuan Yi 1 , Yan Zhang 1 , Qingli He 1 , Dandan Chen 1 , Weiguo Cao 2 , Pengfei Fang 1, 3 , Wen Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-12 , DOI: 10.1021/jacs.2c12507 Yiyuan Cheng 1 , Xuan Yi 1 , Yan Zhang 1 , Qingli He 1 , Dandan Chen 1 , Weiguo Cao 2 , Pengfei Fang 1, 3 , Wen Liu 1
Affiliation
|
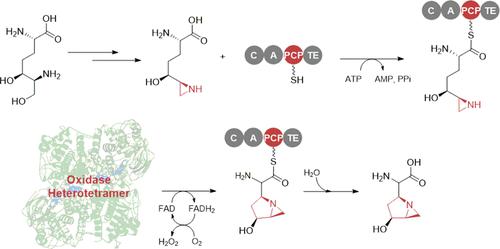
Ficellomycin, azinomycins, and vazabitide A are nonribosomal peptide natural products characterized by an amino acid unit that contains a similar 1-azabicyclo[3.1.0]hexane (ABCH) pharmacophore. This unit is derived from diamino-dihydroxy-heptanic acid (DADH); however, the process through which linear DADH is cyclized to furnish an ABCH ring system remains poorly understood. Based on the reconstitution of the route of the ABCH-containing unit by blending genes/enzymes involved in the biosynthesis of ficellomycin and azinomycins, we report that ABCH formation is completed by an oxidase heterotetramer with the association of a nonribosomal peptide synthetase (NRPS). The DADH precursor was prepared in Escherichia coli to produce a conjugate subjected to in vitro enzymatic hydrolysis for offloading from an amino-group carrier protein. To furnish an aziridine ring, DADH was processed by C7-hydroxyl sulfonation and sulfate elimination-coupled cyclization. Further cyclization leading to an azabicyclic hexane pharmacophore was proved to occur in the NRPS, where the oxidase heterotetramer functions in trans and catalyzes α,β-dehydrogenation to initiate the formation of a fused five-membered nitrogen heterocycle. The identity of ABCH was validated by utilization of the resultant ABCH-containing unit in the total biosynthesis of ficellomycin. Biochemical characterization, crystal structure, and site-specific mutagenesis rationalize the catalytic mechanism of the unusual oxidase heterotetramer.
中文翻译:

氧化酶异四聚体与非核糖体肽合成酶的结合完成 1-氮杂双环 [3.1.0] 己烷的形成
Ficellomycin、azinomycins 和 vazabitide A 是非核糖体肽天然产物,其特征在于氨基酸单元包含类似的 1- a za b i c yclo [3.1.0] h exane (ABCH) 药效团。该单元源自d i a mino- d ihydroxy- h庚酸 (DADH);然而,线性 DADH 环化以提供 ABCH 环系统的过程仍然知之甚少。基于通过混合参与 ficellomycin 和 azinomycins 生物合成的基因/酶重建含 ABCH 单元的路线,我们报告 ABCH 形成是由氧化酶异四聚体与非核糖体肽合成酶 (NRPS) 结合完成的。DADH 前体在大肠杆菌中制备,以产生经过体外处理的缀合物用于从氨基载体蛋白上卸载的酶促水解。为了提供氮丙啶环,DADH 通过 C7-羟基磺化和硫酸盐消除偶联环化处理。进一步环化导致氮杂双环己烷药效团被证明发生在 NRPS 中,其中氧化酶异四聚体在反式中起作用并催化 α,β-脱氢以启动稠合五元氮杂环的形成。通过在 ficellomycin 的总生物合成中使用所得的含 ABCH 单元来验证 ABCH 的身份。生化特征、晶体结构和定点诱变使不寻常的氧化酶异四聚体的催化机制合理化。
更新日期:2023-04-12
中文翻译:

氧化酶异四聚体与非核糖体肽合成酶的结合完成 1-氮杂双环 [3.1.0] 己烷的形成
Ficellomycin、azinomycins 和 vazabitide A 是非核糖体肽天然产物,其特征在于氨基酸单元包含类似的 1- a za b i c yclo [3.1.0] h exane (ABCH) 药效团。该单元源自d i a mino- d ihydroxy- h庚酸 (DADH);然而,线性 DADH 环化以提供 ABCH 环系统的过程仍然知之甚少。基于通过混合参与 ficellomycin 和 azinomycins 生物合成的基因/酶重建含 ABCH 单元的路线,我们报告 ABCH 形成是由氧化酶异四聚体与非核糖体肽合成酶 (NRPS) 结合完成的。DADH 前体在大肠杆菌中制备,以产生经过体外处理的缀合物用于从氨基载体蛋白上卸载的酶促水解。为了提供氮丙啶环,DADH 通过 C7-羟基磺化和硫酸盐消除偶联环化处理。进一步环化导致氮杂双环己烷药效团被证明发生在 NRPS 中,其中氧化酶异四聚体在反式中起作用并催化 α,β-脱氢以启动稠合五元氮杂环的形成。通过在 ficellomycin 的总生物合成中使用所得的含 ABCH 单元来验证 ABCH 的身份。生化特征、晶体结构和定点诱变使不寻常的氧化酶异四聚体的催化机制合理化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号