Food Hydrocolloids ( IF 11.0 ) Pub Date : 2023-04-12 , DOI: 10.1016/j.foodhyd.2023.108769
Liming Miao , Jianyu Zhu , Xinhui Peng , Jianling Feng , Hongxia Dong , Xiaohong Tong , Huan Wang , Lianzhou Jiang
|
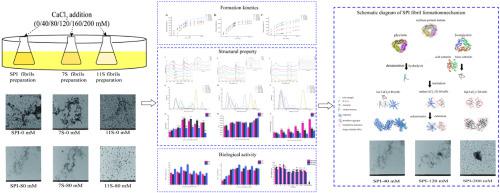
In this study, fibrillation of soybean protein isolate (SPI) and β-conglycinin (7S)/glycinin (11S) in acid-heating with CaCl2 concentration (0–200 mM) was explored. Fibril formation kinetics results showed the fastest fibril formation rate and the most fibrils with 80 mM CaCl2 addition. Fourier-transform infrared (FTIR) spectroscopy, size distribution and Transmission electron microscopy (TEM) results demonstrated SPI fibril structure became more complete and occurred entanglement with CaCl2 concentration increasing from 0 to 80 mM. When CaCl2 addition was above 80 mM, ζ-potential, surface hydrophobicity (H0) and free sulphydryl groups determination proved hydrophobic interaction among fibril built-units was hindered by charges shielded effect, and basic units of 11S were more exposed, aggregate morphology were changed to thicker fibrils or amorphous aggregates. The whole system with CaCl2 has preferable antioxidant property and no cytotoxicity. The study provided a basis for optimizing SPI fibrillation in food industry.
中文翻译:

CaCl2浓度对大豆分离蛋白和β-伴大豆球蛋白/大豆球蛋白原纤维形成及特性的影响
在这项研究中,探索了大豆分离蛋白 (SPI) 和 β-伴大豆球蛋白 (7S)/大豆球蛋白 (11S) 在 CaCl 2浓度 (0–200 mM) 的酸加热条件下的原纤维化。原纤维形成动力学结果显示,添加 80 mM CaCl 2时原纤维形成速度最快,原纤维数量最多。傅里叶变换红外 (FTIR) 光谱、尺寸分布和透射电子显微镜 (TEM) 结果表明,SPI 原纤维结构变得更加完整,并且随着 CaCl 2浓度从 0 增加到 80 mM 发生纠缠。CaCl 2添加量高于80 mM时,ζ电位、表面疏水性( H 0) 和游离巯基测定证明原纤维结构单元之间的疏水相互作用受到电荷屏蔽效应的阻碍,11S 的基本单元暴露更多,聚集体形态变为更粗的原纤维或无定形聚集体。CaCl 2整个体系具有较好的抗氧化性能,无细胞毒性。该研究为优化食品工业中的SPI原纤化提供了依据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号