Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Independent control over cell patterning and adhesion on hydrogel substrates for tissue interface mechanobiology
iScience ( IF 4.6 ) Pub Date : 2023-04-11 , DOI: 10.1016/j.isci.2023.106657 Louis S Prahl 1 , Catherine M Porter 1 , Jiageng Liu 1 , John M Viola 1 , Alex J Hughes 1, 2, 3
iScience ( IF 4.6 ) Pub Date : 2023-04-11 , DOI: 10.1016/j.isci.2023.106657 Louis S Prahl 1 , Catherine M Porter 1 , Jiageng Liu 1 , John M Viola 1 , Alex J Hughes 1, 2, 3
Affiliation
|
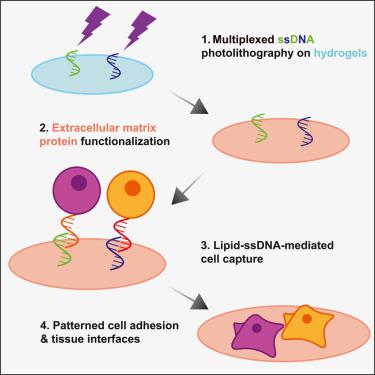
Tissue boundaries and interfaces are engines of morphogenesis in vivo . However, despite a wealth of micropatterning approaches available to control tissue size, shape, and mechanical environment in vitro , fine-scale spatial control of cell positioning within tissue constructs remains an engineering challenge. To address this, we augment DNA “velcro” technology for selective patterning of ssDNA-labeled cells on mechanically defined photoactive polyacrylamide hydrogels. Hydrogels bearing photopatterned single-stranded DNA (ssDNA) features for cell capture are then co-functionalized with extracellular matrix (ECM) proteins to support subsequent adhesion of patterned tissues. ECM protein co-functionalization does not alter ssDNA pattern fidelity, cell capture, or hydrogel elastic stiffness. This approach enables mechanobiology studies and measurements of signaling activity at dynamic cell interfaces with precise initial patterning. Combining DNA velcro patterning and ECM functionalization provides independent control of initial cell placement, adhesion, and mechanics, constituting a new tool for studying biological interfaces and for programming multicellular interactions in engineered tissues.
中文翻译:

独立控制水凝胶基质上的细胞模式和粘附,用于组织界面力学生物学
组织边界和界面是体内形态发生的 引擎。然而,尽管有大量微图案化方法可用于在体外控制组织大小、形状和机械环境 ,但组织构建体内细胞定位的精细空间控制仍然是一项工程挑战。为了解决这个问题,我们增强了 DNA“魔术贴”技术,用于在机械定义的光活性聚丙烯酰胺水凝胶上选择性地模式 ssDNA 标记的细胞。然后,带有用于细胞捕获的光图案化单链 DNA (ssDNA) 特征的水凝胶与细胞外基质 (ECM) 蛋白共功能化,以支持图案化组织的后续粘附。ECM 蛋白共功能化不会改变 ssDNA 模式保真度、细胞捕获或水凝胶弹性刚度。这种方法能够通过精确的初始模式进行机械生物学研究和动态细胞界面信号转导活性的测量。DNA 魔术贴图案和 ECM 功能化相结合,可独立控制初始细胞放置、粘附和力学,构成了研究生物界面和对工程组织中多细胞相互作用进行编程的新工具。
更新日期:2023-04-11
中文翻译:

独立控制水凝胶基质上的细胞模式和粘附,用于组织界面力学生物学
组织边界和界面是体内形态发生的 引擎。然而,尽管有大量微图案化方法可用于在体外控制组织大小、形状和机械环境 ,但组织构建体内细胞定位的精细空间控制仍然是一项工程挑战。为了解决这个问题,我们增强了 DNA“魔术贴”技术,用于在机械定义的光活性聚丙烯酰胺水凝胶上选择性地模式 ssDNA 标记的细胞。然后,带有用于细胞捕获的光图案化单链 DNA (ssDNA) 特征的水凝胶与细胞外基质 (ECM) 蛋白共功能化,以支持图案化组织的后续粘附。ECM 蛋白共功能化不会改变 ssDNA 模式保真度、细胞捕获或水凝胶弹性刚度。这种方法能够通过精确的初始模式进行机械生物学研究和动态细胞界面信号转导活性的测量。DNA 魔术贴图案和 ECM 功能化相结合,可独立控制初始细胞放置、粘附和力学,构成了研究生物界面和对工程组织中多细胞相互作用进行编程的新工具。

































 京公网安备 11010802027423号
京公网安备 11010802027423号