当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strontium and Cesium Adsorption on Exopolysaccharide-Modified Clay Minerals
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-04-11 , DOI: 10.1021/acsearthspacechem.3c00051 Huimin Zhang 1, 2 , Steve Larson 3 , John Ballard 3 , Kauri A. Runge 3 , Jing Nie 1 , Qiqi Zhang 1 , Xianchun Zhu 1 , Nihar Pradhan 1 , Qilin Dai 1 , Youhua Ma 2 , Fengxiang X. Han 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-04-11 , DOI: 10.1021/acsearthspacechem.3c00051 Huimin Zhang 1, 2 , Steve Larson 3 , John Ballard 3 , Kauri A. Runge 3 , Jing Nie 1 , Qiqi Zhang 1 , Xianchun Zhu 1 , Nihar Pradhan 1 , Qilin Dai 1 , Youhua Ma 2 , Fengxiang X. Han 1
Affiliation
|
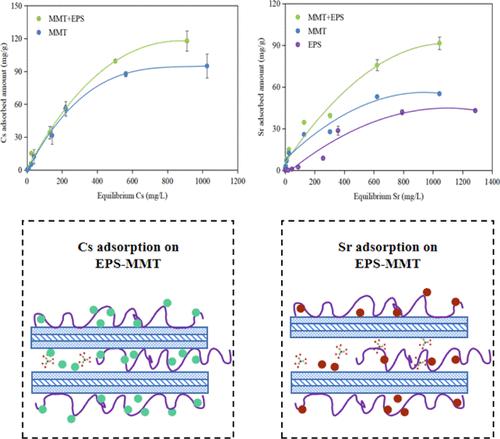
The modification of clay minerals by exopolysaccharides (EPS) may significantly increase their adsorption capacity for heavy metals. This study focused on the adsorption of EPS (produced by Rhizobium tropici)-modified montmorillonite (MMT) and kaolinite (KLT) for Cs and Sr and the influence of external factors (pH, sulfate, and phosphate). The characterization of the composites was carried out using X-ray diffraction (XRD), Fourier transform infrared (FTIR), atomic force microscopy (AFM), and scanning electron microscopy/energy-dispersive X-ray analysis. With EPS modification, the adsorption capacity of MMT for Cs and Sr reached 256 and 90.9 mg/g, respectively, which were significantly improved by 53.8 and 54.5% compared to MMT alone, respectively. The adsorption capacity of KLT for Sr improved by 10.7%. KLT did not adsorb Cs either before or after EPS modification. The adsorption isotherms for Sr on MMT, EPS-MMT, KLT, and EPS-KLT as well as Cs on MMT and EPS-MMT were better described with the Freundlich adsorption models, indicating a heterogeneous layered adsorption process. XRD, FTIR, and AFM analysis confirmed the interlayer reaction of Sr/Cs with EPS-MMT. The Sr amounts adsorbed on EPS-MMT composites increased significantly with increasing pH, while the pH influence was not obvious on Cs adsorption but still slightly increased at pH 7 and then dropped at pH 9. In the presence of 50 and 500 mg/L sulfate, the Sr amount absorbed decreased by 12.5, and 29.3%, respectively. On the contrary, there was a significant increase in Cs adsorption by 12.2 and 33.9%, respectively. In the presence of phosphate, a significant increase (64.5%) was observed for Cs adsorption under 50 mg/L phosphate loading, but 500 mg/L phosphate inhibited (65.8%) the adsorption. In contrast, there was no significant change of Sr adsorption under different phosphate concentrations. The current study would provide a new insight for the application of biopolymers in remediation of Sr- and Cs-contaminated areas.
中文翻译:

锶和铯在胞外多糖改性粘土矿物上的吸附
胞外多糖 (EPS) 对粘土矿物的改性可显着提高其对重金属的吸附能力。本研究的重点是吸附 EPS(由热带根瘤菌产生)-改性蒙脱石 (MMT) 和高岭石 (KLT) 对 Cs 和 Sr 的影响以及外部因素(pH、硫酸盐和磷酸盐)的影响。使用 X 射线衍射 (XRD)、傅立叶变换红外 (FTIR)、原子力显微镜 (AFM) 和扫描电子显微镜/能量色散 X 射线分析对复合材料进行表征。经EPS改性后,MMT对Cs和Sr的吸附量分别达到256和90.9 mg/g,与单独使用MMT相比分别提高了53.8和54.5%。KLT对Sr的吸附能力提高了10.7%。KLT 在 EPS 改性之前或之后均不吸附 Cs。MMT、EPS-MMT、KLT 和 EPS-KLT 上 Sr 的吸附等温线以及 MMT 和 EPS-MMT 上 Cs 的吸附等温线可以用 Freundlich 吸附模型更好地描述,表明非均相层状吸附过程。XRD、FTIR 和 AFM 分析证实了 Sr/Cs 与 EPS-MMT 的层间反应。EPS-MMT 复合材料吸附 Sr 的量随着 pH 的升高而显着增加,而 pH 对 Cs 吸附的影响不明显,但在 pH 7 时仍略有增加,然后在 pH 9 时下降。在 50 和 500 mg/L 硫酸盐存在下, 吸收的 Sr 量分别减少了 12.5% 和 29.3%。相反,Cs 吸附分别显着增加了 12.2% 和 33.9%。在磷酸盐存在下,在 50 mg/L 磷酸盐负载下观察到 Cs 吸附显着增加 (64.5%),但 500 mg/L 磷酸盐抑制 (65.8%) 吸附。相反,在不同磷酸盐浓度下Sr吸附没有显着变化。
更新日期:2023-04-11
中文翻译:

锶和铯在胞外多糖改性粘土矿物上的吸附
胞外多糖 (EPS) 对粘土矿物的改性可显着提高其对重金属的吸附能力。本研究的重点是吸附 EPS(由热带根瘤菌产生)-改性蒙脱石 (MMT) 和高岭石 (KLT) 对 Cs 和 Sr 的影响以及外部因素(pH、硫酸盐和磷酸盐)的影响。使用 X 射线衍射 (XRD)、傅立叶变换红外 (FTIR)、原子力显微镜 (AFM) 和扫描电子显微镜/能量色散 X 射线分析对复合材料进行表征。经EPS改性后,MMT对Cs和Sr的吸附量分别达到256和90.9 mg/g,与单独使用MMT相比分别提高了53.8和54.5%。KLT对Sr的吸附能力提高了10.7%。KLT 在 EPS 改性之前或之后均不吸附 Cs。MMT、EPS-MMT、KLT 和 EPS-KLT 上 Sr 的吸附等温线以及 MMT 和 EPS-MMT 上 Cs 的吸附等温线可以用 Freundlich 吸附模型更好地描述,表明非均相层状吸附过程。XRD、FTIR 和 AFM 分析证实了 Sr/Cs 与 EPS-MMT 的层间反应。EPS-MMT 复合材料吸附 Sr 的量随着 pH 的升高而显着增加,而 pH 对 Cs 吸附的影响不明显,但在 pH 7 时仍略有增加,然后在 pH 9 时下降。在 50 和 500 mg/L 硫酸盐存在下, 吸收的 Sr 量分别减少了 12.5% 和 29.3%。相反,Cs 吸附分别显着增加了 12.2% 和 33.9%。在磷酸盐存在下,在 50 mg/L 磷酸盐负载下观察到 Cs 吸附显着增加 (64.5%),但 500 mg/L 磷酸盐抑制 (65.8%) 吸附。相反,在不同磷酸盐浓度下Sr吸附没有显着变化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号