当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition between Lattice Oxygen and Adsorbate Evolving Mechanisms in Rutile Ru-Based Oxide for the Oxygen Evolution Reaction
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-04-11 , DOI: 10.1021/acsami.3c02086 Shangguo Liu 1 , Yaxiang Chang 1 , Na He 1 , Shenglin Zhu 1 , Lianbao Wang 1 , Xien Liu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-04-11 , DOI: 10.1021/acsami.3c02086 Shangguo Liu 1 , Yaxiang Chang 1 , Na He 1 , Shenglin Zhu 1 , Lianbao Wang 1 , Xien Liu 1
Affiliation
|
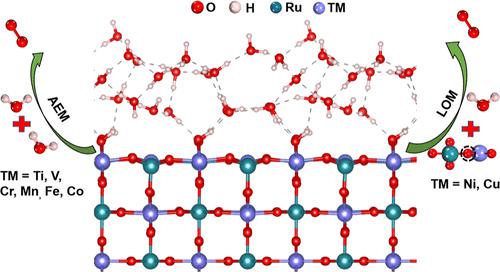
The oxygen evolution reaction (OER) is the primary bottleneck for electrochemical splitting of water into H2. Developing robust and active OER electrocatalysts through understanding the OER mechanism is essential. However, the mechanism for OER is not yet well understood even for the most studied rutile Ru-based oxide, especially in a water-solvent environment. It is still disputed whether the adsorbate evolving mechanism (AEM) is competitive with the lattice oxygen mechanism (LOM). In this article, the AEM and LOM for OER in transition metal (TM)-doped rutile RuO2 with different ratios of TM and Ru are discussed through density functional theory + U calculation. In low TM doping concentration, the evolved O2 is generated through the AEM, and the OER activity is limited by the scaling relationship of OER intermediates. In higher TM doping concentration, the evolved O2 is generated through the LOM for Cu- or Ni-doped RuO2. We find that the distribution of Ru 4d and O 2p orbitals and the adsorption energy of H and O are the major factors that affect the conversion of AEM into LOM. By explicitly considering the water-solvent environment, the LOM can result in higher theoretical OER activity arising from the effects of hydrogen-bond networks.
中文翻译:

金红石钌基氧化物析氧反应中晶格氧与吸附物演化机制的竞争
析氧反应 (OER) 是水电化学分解为 H 2的主要瓶颈。通过了解 OER 机制开发稳健且具有活性的 OER 电催化剂至关重要。然而,即使对于研究最多的金红石 Ru 基氧化物,尤其是在水溶剂环境中,OER 的机制仍未得到很好的理解。吸附物演化机制 (AEM) 是否与晶格氧机制 (LOM) 具有竞争性,目前仍有争议。本文通过密度泛函理论+ U计算讨论了不同TM与Ru比例的过渡金属(TM)掺杂金红石RuO 2中OER的AEM和LOM 。在低 TM 掺杂浓度下,析出的 O 2通过 AEM 生成,OER 活性受限于 OER 中间体的比例关系。在较高的 TM 掺杂浓度下,通过 LOM 为 Cu 或 Ni 掺杂的 RuO 2生成放出的 O 2。我们发现,Ru 4d 和 O 2p 轨道的分布以及 H 和 O 的吸附能是影响 AEM 转化为 LOM 的主要因素。通过明确考虑水溶剂环境,LOM 可以产生更高的理论 OER 活性,这是由氢键网络的影响引起的。
更新日期:2023-04-11
中文翻译:

金红石钌基氧化物析氧反应中晶格氧与吸附物演化机制的竞争
析氧反应 (OER) 是水电化学分解为 H 2的主要瓶颈。通过了解 OER 机制开发稳健且具有活性的 OER 电催化剂至关重要。然而,即使对于研究最多的金红石 Ru 基氧化物,尤其是在水溶剂环境中,OER 的机制仍未得到很好的理解。吸附物演化机制 (AEM) 是否与晶格氧机制 (LOM) 具有竞争性,目前仍有争议。本文通过密度泛函理论+ U计算讨论了不同TM与Ru比例的过渡金属(TM)掺杂金红石RuO 2中OER的AEM和LOM 。在低 TM 掺杂浓度下,析出的 O 2通过 AEM 生成,OER 活性受限于 OER 中间体的比例关系。在较高的 TM 掺杂浓度下,通过 LOM 为 Cu 或 Ni 掺杂的 RuO 2生成放出的 O 2。我们发现,Ru 4d 和 O 2p 轨道的分布以及 H 和 O 的吸附能是影响 AEM 转化为 LOM 的主要因素。通过明确考虑水溶剂环境,LOM 可以产生更高的理论 OER 活性,这是由氢键网络的影响引起的。































 京公网安备 11010802027423号
京公网安备 11010802027423号