Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-04-10 , DOI: 10.1016/j.actbio.2023.04.002 Mengjie Kong 1 , Yan Peng 1 , Liyan Qiu 1
|
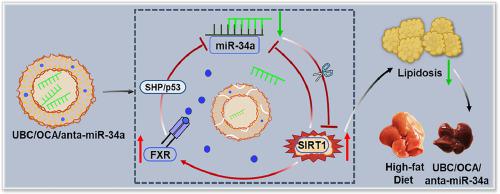
Non-alcoholic fatty liver disease (NAFLD) is currently a common chronic liver disease worldwide. By now, however, there isn't any FDA-approved specific drug for NAFLD treatment. It has been noticed that farnesoid X receptor (FXR), miR-34a and Sirtuin1 (SIRT1) is related to the occurrence and development of NAFLD. A oligochitosan-derivated nanovesicle (UBC) with esterase responsive degradability was designed to co-encapsulate FXR agonist (obeticholic acid, OCA) and miR-34a antagomir (anta-miR-34a) into the hydrophobic membrane and the center aqueous lumen of nanovesicles, respectively, by dialysis method. The action of UBC/OCA/anta-miR-34a loop on the regulation of lipid deposition via nanovesicles was evaluated on high-fat HepG2 cells and HFD-induced mice. The obtained dual drug-loaded nanovesicles UBC/OCA/anta-miR-34a could enhance the cellular uptake and intracellular release of OCA and anta-miR-34a, leading to the reduced lipid deposition in high-fat HepG2 cells. In NAFLD mice models, UBC/OCA/anta-miR-34a achieved the best curative effect on the recovery of body weight and hepatic function. Meanwhile, in vitro and vivo experiments validated that UBC/OCA/anta-miR-34a effectively activated the expression level of SIRT1 by enhancing the FXR/miR-34a/SIRT1 regulatory loop. This study provides a promising strategy for constructing oligochitosan-derivated nanovesicles to co-deliver OCA and anta-miR-34a for NAFLD treatment.
Statement of significance
This study proposed a strategy to construct oligochitosan-derivated nanovesicles to co-deliver obeticholic acid and miR-34a antagomir for NAFLD treatment. Based on the FXR/miR-34a/SIRT1 action loop, this nanovesicle effectively exerted a synergetic effect of OCA and anta-miR-34a to significantly regulate lipid deposition and recover liver function in NAFLD mice.
中文翻译:

基于壳寡糖的纳米囊泡通过 FXR/miR-34a/SIRT1 调节环治疗非酒精性脂肪肝
非酒精性脂肪性肝病(NAFLD)是目前世界范围内常见的慢性肝病。然而,到目前为止,还没有任何 FDA 批准的治疗 NAFLD 的特异性药物。已经注意到法尼醇X受体(FXR)、miR-34a和Sirtuin1(SIRT1)与NAFLD的发生发展有关。一种具有酯酶响应降解能力的壳寡糖衍生纳米囊泡 (UBC)被设计用于共包封 FXR 激动剂(奥贝胆酸,OCA)和 miR-34a antagomir(anta-miR-34a) 通过透析法分别进入纳米囊泡的疏水膜和中心水腔。在高脂肪 HepG2 细胞和 HFD 诱导的小鼠上评估了 UBC/OCA/anta-miR-34a 环通过纳米囊泡调节脂质沉积的作用。获得的双载药纳米囊泡 UBC/OCA/anta-miR-34a 可以增强 OCA 和 anta-miR-34a 的细胞摄取和细胞内释放,从而减少高脂肪 HepG2 细胞中的脂质沉积。在NAFLD小鼠模型中,UBC/OCA/anta-miR-34a对体重和肝功能的恢复取得了最好的疗效。同时,体外和体内实验验证了 UBC/OCA/anta-miR-34a 通过增强 FXR/miR-34a/SIRT1 调节环有效激活了 SIRT1 的表达水平。该研究为构建寡聚壳聚糖衍生的纳米囊泡以共同递送 OCA 和 anta-miR-34a 用于 NAFLD 治疗提供了一种有前途的策略。
重要性声明
本研究提出了一种构建寡聚壳聚糖衍生的纳米囊泡的策略,以共同递送奥贝胆酸和 miR-34a 拮抗剂用于 NAFLD 治疗。基于 FXR/miR-34a/SIRT1 作用环,该纳米囊泡有效发挥 OCA 和 anta-miR-34a 的协同作用,显着调节 NAFLD 小鼠的脂质沉积和恢复肝功能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号