当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
氢键与氢化氢的氢键——实验低温红外和计算研究:氢键的修订定义是否合适?
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-10 , DOI: 10.1021/jacs.3c00802
Svatopluk Civiš 1, 2 , Maximilián Lamanec 1, 3, 4 , Vladimír Špirko 1 , Jiří Kubišta 2 , Matej Špet'ko 3 , Pavel Hobza 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-10 , DOI: 10.1021/jacs.3c00802
Svatopluk Civiš 1, 2 , Maximilián Lamanec 1, 3, 4 , Vladimír Špirko 1 , Jiří Kubišta 2 , Matej Špet'ko 3 , Pavel Hobza 1, 3
Affiliation
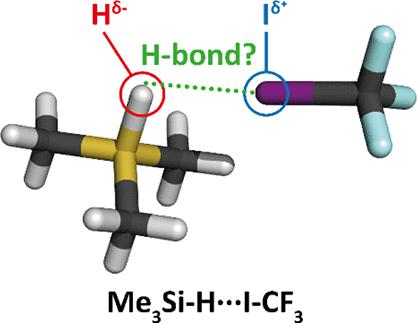
|
Me 3 Si–H···Y配合物的光谱特征(Y = ICF 3, BrCN, and HCN) containing a hydridic hydrogen is experimentally determined by low-temperature IR experiments based on the direct spectral measurement of ultrasonic expanded intermediates on a cold substrate or by the technique of argon-matrix isolation as well as computationally at harmonic and one -维度非谐波水平。计算基于使用各种扩展 AO 基组的 DFT-D、MP2、MP2-F12 和 CCSD(T)-F12 水平。所有与复合物形成时 Si-H 伸缩频率红移相关的复合物的形成都伴随着其强度的增加。对于另外 10 个不同类型的电子受体、正 σ-、π- 和 p-空穴和阳离子,也获得了类似的结果。HBe-H···Y 配合物的形成,仅通过计算研究并再次包含氢,其特征是 Be-H 伸缩频率在络合时发生蓝移,并伴随着强度的增加。目前研究的所有氢氢键合配合物的光谱偏移和稳定能量都与质子氢键配合物相当,这促使我们提出修改现有的 IUPAC 氢键定义,除了经典的质子形式,非经典的氢和二氢形式。

"点击查看英文标题和摘要"
更新日期:2023-04-10

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号