Phytochemistry ( IF 3.2 ) Pub Date : 2023-04-09 , DOI: 10.1016/j.phytochem.2023.113672 Hermine Wete Nono 1 , Arno Rusel Donfack Nanfack 2 , Billy Toussie Tchegnitegni 2 , Cyrille Armel Njanpa Ngansop 3 , Faustine Léonie Mafodong Dongmo 4 , Maurice Ducret Awouafack 2 , Fabrice Fekam Boyom 3 , Bruno Lenta Ndjakou 5 , Hans-Georg Stammler 6 , Beate Neumann 6 , Norbert Sewald 7 , Silvère Augustin Ngouela 1
|
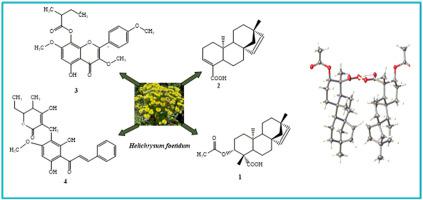
The phytochemical investigation of the MeOH and CH2Cl2–MeOH (1:1) extracts from the flowers and twigs of Helichrysum foetidum (L.) Moench (Asteraceae), which showed antileishmanial and antiplasmodial activities during the preliminary screening, led to the isolation of four undescribed compounds, including two ent-beyer-15-ene-type diterpenoids, foetidumins A (1) and B (2), one flavonoid, foetidumin C (3) and one chalcopyrone, foetidumin D (4). Additionally, fourteen known compounds comprising, two ent-beyer-15-ene-type diterpenoids (5–6), six flavonoids (7–12), two steroids (13–14), three triterpenoids (15–17), and one glyceryl monostearate (18) were also isolated. The chemical structures of foetidumins A-D were fully elucidated by analyses of their spectroscopic data. The structure and the stereochemistry of foetidumin A (1) were confirmed by SC-XRD analyses. Among the tested compounds, foetidumin C (3), erythroxylol A (6), and kaempferol (7) displayed the highest antileishmanial potency with IC50 values of 13.0, 11.8, and 11.1 μM, respectively. Foetidumin C (3) had no cytotoxicity toward Vero cells with the selectivity index > 3.59. Meanwhile, extracts of flowers and twigs had higher activity against Plasmodium falciparum chloroquine-sensitive (Pf3D7) strain with IC50 values of 3.66 and 10.52 μg/mL, respectively.
中文翻译:

Foetidumins AD,以及来自 Helichrysum foetidum (L.) Moench (Asteraceae) 具有抗寄生虫活性的其他化学成分
对从Helichrysum foetidum (L.) Moench(菊科)的花和枝条中提取的MeOH 和 CH 2 Cl 2 –MeOH (1:1)提取物的植物化学研究表明,在初步筛选过程中显示出抗犬瘟热和抗疟原虫活性,导致了分离出四种未描述的化合物,包括两种ent -beyer-15-ene 型二萜类化合物 foetidumins A ( 1 ) 和 B ( 2 ),一种类黄酮 foetidumin C ( 3 ) 和一种查尔考酮 foetidumin D ( 4 )。此外,十四种已知化合物包括两种ent -beyer-15-ene 型二萜 ( 5-6 )、六种类黄酮 ( 7-12)、两种类固醇(13-14 )、三种三萜类化合物(15-17 )和一种单硬脂酸甘油酯(18 )也被分离出来。foetidumins AD 的化学结构通过对其光谱数据的分析得到了充分阐明。foetidumin A ( 1 ) 的结构和立体化学通过 SC-XRD 分析确认。在测试的化合物中,foetidumin C ( 3 )、erythroxylol A ( 6 ) 和山奈酚 ( 7 ) 显示出最高的抗利什曼原虫效力,IC 50值分别为 13.0、11.8 和 11.1 μM。Foetidumin C ( 3 )对Vero细胞无细胞毒性选择性指数 > 3.59。同时,花和嫩枝提取物对恶性疟原虫氯喹敏感( Pf 3D7)菌株具有较高的活性,IC 50值分别为3.66和10.52 μg/mL。

































 京公网安备 11010802027423号
京公网安备 11010802027423号