European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-04-06 , DOI: 10.1016/j.ejmech.2023.115286 Andrea Galbiati 1 , Stefania Bova 2 , Raffaella Pacchiana 3 , Chiara Borsari 4 , Marco Persico 5 , Aureliano Zana 1 , Stefano Bruno 6 , Massimo Donadelli 3 , Caterina Fattorusso 5 , Paola Conti 1
|
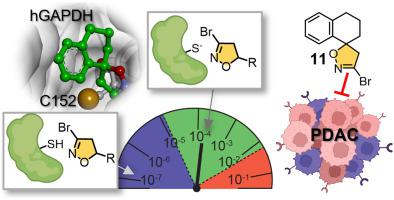
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a key glycolytic enzyme, plays a crucial role in the energy metabolism of cancer cells and has been proposed as a valuable target for the development of anticancer agents. Among a series of 5-substituted 3-bromo-4,5-dihydroisoxazole (BDHI) derivatives, we identified the spirocyclic compound 11, which is able to covalently inactivate recombinant human GAPDH (hGAPDH) with a faster reactivity than koningic acid, one of the most potent hGAPDH inhibitors known to date. Computational studies confirmed that conformational rigidification is crucial to stabilize the interaction of the inhibitor with the binding site, thus favoring the subsequent covalent bond formation. Investigation of intrinsic warhead reactivity at different pH disclosed the negligible reactivity of 11 with free thiols, highlighting its ability to selectively react with the activated cysteine of hGAPDH with respect to other sulfhydryl groups. Compound 11 strongly reduced cancer cell growth in four different pancreatic cancer cell lines and its antiproliferative activity correlated well with the intracellular inhibition of hGAPDH. Overall, our results qualify 11 as a potent hGAPDH covalent inhibitor with a moderate drug-like reactivity that could be further exploited to develop anticancer agents.
中文翻译:

发现具有抗胰腺癌细胞增殖活性的 hGAPDH 螺环 3-溴-4,5-二氢异恶唑共价抑制剂
3-磷酸甘油醛脱氢酶(GAPDH)是一种关键的糖酵解酶,在癌细胞的能量代谢中发挥着至关重要的作用,并被认为是抗癌药物开发的一个有价值的靶点。在一系列 5-取代的 3-溴-4,5-二氢异恶唑 (BDHI) 衍生物中,我们鉴定了螺环化合物11 ,它能够共价灭活重组人 GAPDH (hGAPDH),其反应活性比康宁酸 (koningic Acid) 更快。迄今为止已知最有效的 hGAPDH 抑制剂。计算研究证实,构象刚性对于稳定抑制剂与结合位点的相互作用至关重要,从而有利于随后的共价键形成。对不同pH下弹头内在反应性的研究揭示了11与游离硫醇的反应性可以忽略不计,突出了其相对于其他巯基选择性地与hGAPDH的活化半胱氨酸反应的能力。化合物11强烈降低四种不同胰腺癌细胞系中癌细胞的生长,其抗增殖活性与 hGAPDH 的细胞内抑制密切相关。总体而言,我们的结果表明11是一种有效的 hGAPDH 共价抑制剂,具有中等的药物样反应活性,可进一步用于开发抗癌药物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号