当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective activation of peroxymonosulfate govern by B-site metal in delafossite for efficient pollutants degradation: Pivotal role of d orbital electronic configuration
Water Research ( IF 11.4 ) Pub Date : 2023-04-08 , DOI: 10.1016/j.watres.2023.119957 Ying Zhao 1 , Shixuan Chen 1 , Hang Qie 1 , Shishu Zhu 2 , Changyong Zhang 3 , Xueyan Li 4 , Wei Wang 1 , Jun Ma 1 , Zhiqiang Sun 1
Water Research ( IF 11.4 ) Pub Date : 2023-04-08 , DOI: 10.1016/j.watres.2023.119957 Ying Zhao 1 , Shixuan Chen 1 , Hang Qie 1 , Shishu Zhu 2 , Changyong Zhang 3 , Xueyan Li 4 , Wei Wang 1 , Jun Ma 1 , Zhiqiang Sun 1
Affiliation
|
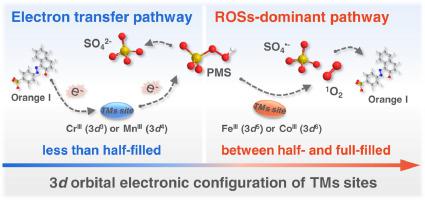
Radical and non-radical oxidation pathways have been universally validated in transition metals (TMs) oxides activated peroxymonosulfate (PMS) processes. However, achieving high efficiency and selectivity of PMS activation remains challenging due to the ambiguous tuning mechanism of TMs sites on PMS activation in thermodynamic scope. Herein, we demonstrated that the exclusive PMS oxidation pathways were regulated by orbital electronic configuration of B-sites in delafossites (CuBO) for Orange I degradation (Co 3 for reactive oxygen species (ROSs) vs. Cr 3 for electron transfer pathway). The orbital electronic configuration was identified to affect the orbital overlap extent between 3 of B-sites and O 2 of PMS, which induced B-sites offering different types of hybrid orbital to coordinate with O 2 of PMS, thereby forming the high-spin complex (CuCoO@PMS) or the low-spin complex (CuCrO@PMS), on which basis PMS was selectively dissociated to form ROSs or achieve electron transfer pathway. As indicated by thermodynamic analysis, a general rule was proposed that B-sites of less than half-filled 3 orbital tended to act as electron shuttle, i.e., Cr (3), Mn (3), interacting with PMS to execute an electron transfer pathway for degrading Orange I, while B-sites of between half-filled and full-filled 3 orbital preferred to be electron donator, i.e., Co (3), Fe (3), activating PMS to generate ROSs. These findings lay a foundation for the oriented design of TMs-based catalysts from the atomic level according to orbital electronic configuration optimization, as so to facilitate the achievement of PMS-AOPs with highly selective and efficient remediation of contaminants in water purification practice.
中文翻译:

铜铁矿中 B 位金属控制的过一硫酸盐选择性活化,有效降解污染物:d 轨道电子构型的关键作用
自由基和非自由基氧化途径已在过渡金属 (TM) 氧化物激活的过一硫酸盐 (PMS) 工艺中得到普遍验证。然而,由于热力学范围内TMs位点对PMS激活的调节机制不明确,实现PMS激活的高效率和选择性仍然具有挑战性。在此,我们证明了独特的 PMS 氧化途径受到铜铁矿 (CuBO) 中 B 位点轨道电子构型的调节,以实现 Orange I 降解(Co 3 用于活性氧物种 (ROS),而 Cr 3 用于电子转移途径)。确定轨道电子构型影响3个B位点与PMS的O 2 之间的轨道重叠程度,从而诱导B位点提供不同类型的混合轨道与PMS的O 2 配位,从而形成高自旋配合物(CuCoO@PMS)或低自旋配合物(CuCrO@PMS),在此基础上PMS选择性解离形成ROS或实现电子传递途径。热力学分析表明,一般规则是小于半满3轨道的B位倾向于充当电子穿梭者,即Cr(3)、Mn(3),与PMS相互作用以执行电子转移降解Orange I的途径,而半填充和全填充3轨道之间的B位优选为电子供体,即Co(3)、Fe(3),激活PMS产生ROS。这些发现为根据轨道电子构型优化从原子水平定向设计基于TMs的催化剂奠定了基础,从而促进在水净化实践中实现高选择性和高效污染物修复的PMS-AOPs。
更新日期:2023-04-08
中文翻译:

铜铁矿中 B 位金属控制的过一硫酸盐选择性活化,有效降解污染物:d 轨道电子构型的关键作用
自由基和非自由基氧化途径已在过渡金属 (TM) 氧化物激活的过一硫酸盐 (PMS) 工艺中得到普遍验证。然而,由于热力学范围内TMs位点对PMS激活的调节机制不明确,实现PMS激活的高效率和选择性仍然具有挑战性。在此,我们证明了独特的 PMS 氧化途径受到铜铁矿 (CuBO) 中 B 位点轨道电子构型的调节,以实现 Orange I 降解(Co 3 用于活性氧物种 (ROS),而 Cr 3 用于电子转移途径)。确定轨道电子构型影响3个B位点与PMS的O 2 之间的轨道重叠程度,从而诱导B位点提供不同类型的混合轨道与PMS的O 2 配位,从而形成高自旋配合物(CuCoO@PMS)或低自旋配合物(CuCrO@PMS),在此基础上PMS选择性解离形成ROS或实现电子传递途径。热力学分析表明,一般规则是小于半满3轨道的B位倾向于充当电子穿梭者,即Cr(3)、Mn(3),与PMS相互作用以执行电子转移降解Orange I的途径,而半填充和全填充3轨道之间的B位优选为电子供体,即Co(3)、Fe(3),激活PMS产生ROS。这些发现为根据轨道电子构型优化从原子水平定向设计基于TMs的催化剂奠定了基础,从而促进在水净化实践中实现高选择性和高效污染物修复的PMS-AOPs。































 京公网安备 11010802027423号
京公网安备 11010802027423号