Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-04-07 , DOI: 10.1016/j.cclet.2023.108434 Jingwen Zhang , Jiahui Yan , Yanan Wang , Hong Liu , Xueping Sun , Yuchao Gu , Liangmin Yu , Changcheng Li , Jun Wu , Zhiyu He
|
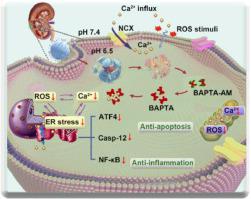
Early pathogenesis of ischemia-reperfusion (I/R)-induced acute kidney injury (AKI) is dominated by intracellular calcium overload, which induces oxidative stress, intracellular energy metabolism disorder, inflammatory activation, and a series of pathologic cascaded reactions that are closely intertwined with self-amplifying and interactive feedback loops, ultimately resulting in cell damage and kidney failure. Currently, most nanomedicines originate from the perspective of antioxidant stress, which can only quench existing reactive oxide species (ROS) but cannot prevent the continuous production of ROS, resulting in insufficient efficacy. As a safe and promising drug, BAPTA-AM is hydrolyzed into BAPTA by intracellular esterase upon entering cells, which can rapidly chelate with overloaded Ca2+, restoring intracellular calcium homeostasis, thus inhibiting ROS regeneration at the source. Here, we designed a KTP-targeting peptide-modified yolk-shell structure of liposome–poly(ethylene glycol)methyl ether-block-poly (l-lactide-co-glycolic) (mPLGA) hybrid nanoparticles (<100 nm), with the characteristics of high encapsulation rate, high colloid stability, facile modification, and prolonged blood circulation time. Once the BA/mPLGA@Lipo-KTP was targeted to the site of kidney injury, the cholesteryl hemisuccinate (CHEMS) in the phospholipid bilayer, as an acidic cholesterol ester, was protonated in the simulated inflammatory slightly acidic environment (pH 6.5), causing the liposomes to rupture and release the BA/mPLGA nanoparticles, which were then depolymerized by intracellular esterase. The BAPTA-AM was diffused and hydrolyzed to produce BAPTA, which can rapidly cut off the malignant loop of calcium overload/ROS generation at its source, blocking the endoplasmic reticulum (ER) apoptosis pathway (ATF4–CHOP–Bax/Bcl-2, Casp-12–Casp-3) and the inflammatory pathway (TNF-α–NF-κB–IL-6 axes), thus alleviating pathological changes in kidney tissue, thereby inhibiting the expression of renal tubular marker kidney injury molecule 1 (Kim-1) (reduced by 82.9%) and also exhibiting prominent anti-apoptotic capability (TUNEL-positive ratio decreased from 40.2% to 8.3%), significantly restoring renal function. Overall, this research holds huge potential in the treatment of I/R injury-related diseases.
中文翻译:

1,2-双(2-氨基苯氧基)乙烷-N,N,N',N'-四乙酸乙酰氧基甲酯酯负载脂质-mPLGA杂化纳米颗粒恢复细胞钙稳态以缓解内质网应激,用于急性肾损伤治疗
缺血再灌注(I/R)诱发的急性肾损伤(AKI)的早期发病机制以细胞内钙超载为主,引起氧化应激、细胞内能量代谢紊乱、炎症激活等一系列紧密相连的病理级联反应具有自我放大和交互式反馈循环,最终导致细胞损伤和肾衰竭。目前,大多数纳米药物都是从抗氧化应激的角度出发,只能淬灭现有的活性氧化物(ROS),而无法阻止ROS的持续产生,导致疗效不足。作为一种安全、有前景的药物,BAPTA-AM进入细胞后被细胞内酯酶水解为BAPTA,能与超载的Ca2+,恢复细胞内钙稳态,从而从源头抑制ROS再生。在这里,我们设计了一种KTP靶向肽修饰的脂质体-聚(乙二醇)甲醚-嵌段-聚(l-丙-共-乙醇)(mPLGA)混合纳米粒子(<100 nm)的蛋黄壳结构,具有包封率高、胶体稳定性高、易于改性、延长血液循环时间等特点。一旦 BA/mPLGA@Lipo-KTP 靶向肾损伤部位,磷脂双层中的胆固醇半琥珀酸酯 (CHEMS) 作为酸性胆固醇酯,在模拟炎症微酸性环境(pH 6.5)中质子化,导致脂质体破裂并释放 BA/mPLGA 纳米颗粒,然后通过细胞内酯酶解聚。BAPTA-AM扩散水解产生BAPTA,可快速从源头上切断钙超载/ROS生成的恶性循环,阻断内质网(ER)凋亡途径(ATF4–CHOP–Bax/Bcl-2, Casp-12-Casp-3)和炎症通路(TNF- α-NF-κB-IL-6轴),从而减轻肾组织的病理变化,从而抑制肾小管标志物肾损伤分子1(Kim -1)(减少82.9%),并表现出显着的抗凋亡能力(TUNEL阳性率从40.2%下降至8.3%),显着恢复肾功能。总体而言,这项研究在治疗 I/R 损伤相关疾病方面具有巨大潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号