Journal of Ethnopharmacology ( IF 4.8 ) Pub Date : 2023-04-07 , DOI: 10.1016/j.jep.2023.116451 Fanxing Xu 1 , Jingxian Wu 2 , Yumei Hu 3 , Chun Chu 4 , Wenjun Liu 3 , Xiang Li 5 , Wen Zheng 2 , Weishuo Yang 2 , Boyan Zhao 2 , Jiangxue Guo 2 , Zhenzhong Wang 3 , Ying Jia 6 , Wei Xiao 3
|
Ethnopharmacological relevance
Tongsaimai (TSM) is a traditional Chinese medicine that has several therapeutic qualities, including anti-inflammatory, anti-oxidative, and anti-vasculitis effects. However, its impacts and underlying mechanisms on wound healing remain unclear.
Aim of the study
The aim of our study was to evaluate TSM for its pro-healing effect and the relevant mechanisms using both experimental validation and network pharmacology analysis.
Materials and methods
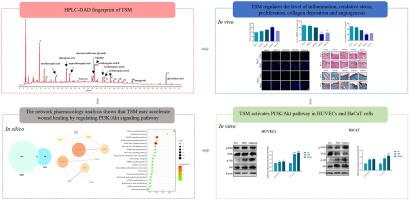
The components of TSM were detected by high-performance liquid chromatography combined with diode array detector (HPLC-DAD). Skin wounds with a diameter of 4 mm were created on the backs of mice, after which, topical treatments of 2.5–10% TSM were applied onto the lesions once daily for either 2 or 7 days. Then, the wound tissues were collected to determine the impacts of TSM on collagen deposition, epithelial cell proliferation, oxidative stress, inflammation, and angiogenesis. Moreover, the effects of TSM (0.5–2 mg/mL) on the cell viability of HUVECs and HaCaT cells were evaluated.
Results
A total of 11 components in TSM were identified by HPLC-DAD. TSM was found to enhance the rate of wound contraction and increase epithelial thickness and collagen deposition during the healing process. In addition, TSM increased SOD activity and downregulated MDA and IL-1β levels in the wound tissues. Immunofluorescence analysis further indicated an increased expression of Ki67, CD31, and VEGF in wound tissues following TSM administration. Results of the network pharmacology analysis revealed that multiple pathways including VEGF, PI3K/Akt, and MAPK pathways were involved in the pharmacological actions of TSM on wound healing. Accordantly, in vitro experiments revealed that TSM promoted the proliferation of HUVECs and HaCaT cells while activating the PI3K/Akt pathway.
Conclusions
Our results suggest that TSM may serve as a therapeutic medication to improve wound healing by employing multiple regulatory mechanisms that affect proliferation, angiogenesis, collagen deposition, oxidative stress, and inflammation.
中文翻译:

基于实验和网络药理学的通塞脉促进伤口愈合的作用机制
民族药理学相关性
通塞麦 (TSM) 是一种传统中药,具有多种治疗功效,包括抗炎、抗氧化和抗血管炎作用。然而,其对伤口愈合的影响和潜在机制仍不清楚。
研究目的
我们研究的目的是使用实验验证和网络药理学分析来评估 TSM 的促愈合作用和相关机制。
材料和方法
TSM的成分采用高效液相色谱结合二极管阵列检测器(HPLC-DAD)进行检测。在小鼠背部造成直径为 4 毫米的皮肤伤口,之后,将 2.5-10% TSM 的局部治疗应用于病变,每天一次,持续 2 或 7 天。然后,收集伤口组织以确定 TSM 对胶原沉积、上皮细胞增殖、氧化应激、炎症和血管生成的影响。此外,还评估了 TSM (0.5–2 mg/mL) 对 HUVEC 和 HaCaT 细胞的细胞活力的影响。
结果
通过 HPLC-DAD 鉴定了 TSM 中的 11 种成分。发现 TSM 可在愈合过程中提高伤口收缩率并增加上皮厚度和胶原蛋白沉积。此外,TSM 增加了 SOD 活性并下调了伤口组织中的MDA和 IL-1β 水平。免疫荧光分析进一步表明,在施用 TSM 后伤口组织中 Ki67、CD31 和 VEGF 的表达增加。网络药理学分析结果显示,包括 VEGF、PI3K/Akt 和 MAPK 通路在内的多条通路参与了 TSM 对伤口愈合的药理作用。因此,体外实验表明,TSM 在激活 PI3K/Akt 通路的同时促进 HUVEC 和 HaCaT 细胞的增殖。
结论
我们的结果表明,TSM 可以作为一种治疗药物,通过采用影响增殖、血管生成、胶原沉积、氧化应激和炎症的多种调节机制来改善伤口愈合。































 京公网安备 11010802027423号
京公网安备 11010802027423号