Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Low-Concentration Redox-Electrolytes for High-Rate and Long-Life Zinc Metal Batteries
Small ( IF 13.0 ) Pub Date : 2023-04-07 , DOI: 10.1002/smll.202207664 Shipeng Wang 1 , Yuwei Zhao 2 , Haiming Lv 2 , Xuanhe Hu 1 , Jun He 1 , Chunyi Zhi 2 , Hongfei Li 2, 3
Small ( IF 13.0 ) Pub Date : 2023-04-07 , DOI: 10.1002/smll.202207664 Shipeng Wang 1 , Yuwei Zhao 2 , Haiming Lv 2 , Xuanhe Hu 1 , Jun He 1 , Chunyi Zhi 2 , Hongfei Li 2, 3
Affiliation
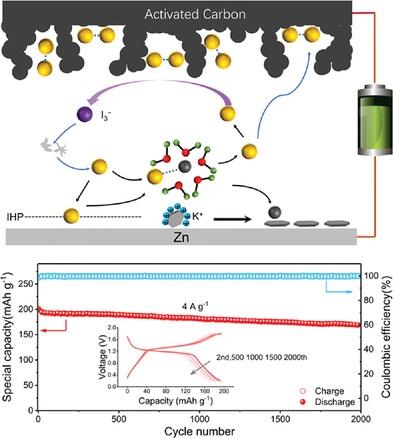
|
The uncontrolled zinc electrodeposition and side reactions severely limit the power density and lifespan of Zn metal batteries. Herein, the multi-level interface adjustment effect is realized with low-concentration redox-electrolytes (0.2 m KI) additives. The iodide ions adsorbed on the zinc surface significantly suppress water-induced side reactions and by-product formation and enhance the kinetics of zinc deposition. The distribution of relaxation times results reveal that iodide ions can reduce the desolvation energy of hydrated zinc ions and guide the deposition of zinc ions due to their strong nucleophilicity. As a consequence, the Zn||Zn symmetric cell achieves superior cycling stability (>3000 h at 1 mA cm−2, 1 mAh cm−2) accompanied by a uniform deposition and a fast reaction kinetics with a low voltage hysteresis (<30 mV). Additionally, coupled with an activated carbon (AC) cathode, the assembled Zn||AC cell delivers a high-capacity retention of 81.64% after 2000 cycles at 4 A g−1. More importantly, the operando electrochemical UV–vis spectroscopies show that a small number of I3− can spontaneously react with the dead zinc as well as basic zinc saltsand regenerate iodide ions and zinc ions; thus, the Coulombic efficiency of each charge–discharge process is close to 100%.
中文翻译:

用于高倍率和长寿命锌金属电池的低浓度氧化还原电解质
不受控制的锌电沉积和副反应严重限制了锌金属电池的功率密度和寿命。在此,多级界面调节效果是通过低浓度氧化还原电解质(0.2 m KI)添加剂实现的。吸附在锌表面的碘离子显着抑制了水引起的副反应和副产物的形成,并增强了锌沉积的动力学。弛豫时间分布结果表明,碘离子可以降低水合锌离子的去溶剂化能,并由于其强亲核性而引导锌离子的沉积。因此,Zn||Zn 对称电池实现了出色的循环稳定性(在 1 mA cm -2、1 mAh cm -2下 >3000 h) 伴随着均匀的沉积和具有低电压滞后 (<30 mV) 的快速反应动力学。此外,与活性炭 (AC) 阴极相结合,组装的 Zn||AC 电池在 4 A g -1 下经过 2000 次循环后可提供 81.64% 的高容量保持率。更重要的是,原位电化学紫外-可见光谱表明,少量的 I 3 -可以自发地与死锌以及碱性锌盐发生反应,并再生碘离子和锌离子;因此,每个充放电过程的库仑效率接近 100%。
更新日期:2023-04-08
中文翻译:

用于高倍率和长寿命锌金属电池的低浓度氧化还原电解质
不受控制的锌电沉积和副反应严重限制了锌金属电池的功率密度和寿命。在此,多级界面调节效果是通过低浓度氧化还原电解质(0.2 m KI)添加剂实现的。吸附在锌表面的碘离子显着抑制了水引起的副反应和副产物的形成,并增强了锌沉积的动力学。弛豫时间分布结果表明,碘离子可以降低水合锌离子的去溶剂化能,并由于其强亲核性而引导锌离子的沉积。因此,Zn||Zn 对称电池实现了出色的循环稳定性(在 1 mA cm -2、1 mAh cm -2下 >3000 h) 伴随着均匀的沉积和具有低电压滞后 (<30 mV) 的快速反应动力学。此外,与活性炭 (AC) 阴极相结合,组装的 Zn||AC 电池在 4 A g -1 下经过 2000 次循环后可提供 81.64% 的高容量保持率。更重要的是,原位电化学紫外-可见光谱表明,少量的 I 3 -可以自发地与死锌以及碱性锌盐发生反应,并再生碘离子和锌离子;因此,每个充放电过程的库仑效率接近 100%。












































 京公网安备 11010802027423号
京公网安备 11010802027423号