当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Hydrogen Bonds on CO2 Capture by Functionalized Deep Eutectic Solvents Derived from 4-Fluorophenol
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-04-07 , DOI: 10.1021/acssuschemeng.2c07590 Zonghua Wang 1 , Mingzhe Chen 1 , Bohao Lu 1 , Shaoze Zhang 2, 3 , Dezhong Yang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-04-07 , DOI: 10.1021/acssuschemeng.2c07590 Zonghua Wang 1 , Mingzhe Chen 1 , Bohao Lu 1 , Shaoze Zhang 2, 3 , Dezhong Yang 1
Affiliation
|
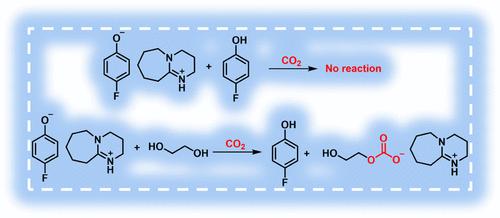
Deep eutectic solvents (DESs) have received a great amount of attention for CO2 uptake due to their unique properties. Here, deep eutectic solvents (DESs) based on 4-fluorophenol-derived superbase ionic liquid are studied for CO2 capture. The ionic liquid used is [DBUH][4-F-PhO], formed by 1,8-diazabicyclo[5.4.0]undecane-7-ene (DBU) and 4-fluorophenol (4-F-PhOH). The DESs are obtained by mixing [DBUH][4-F-PhO] with ethylene glycol (EG) or 4-F-PhOH. Surprisingly, [DBUH][4-F-PhO]-EG DESs present a much higher CO2 capacity (∼1.0 mol CO2/mol solvent) than [DBUH][4-F-PhO]-4-F-PhOH (∼0.10 mol CO2/mol solvent) at 25 °C and 1.0 atm. However, after EG is added into [DBUH][4-F-PhO]-4-F-PhOH, the ternary solvents [DBUH][4-F-PhO]-4-F-PhOH-EG exhibit an unexpected high capacity, although both EG and [DBUH][4-F-PhO]-4-F-PhOH exhibit a low capacity. Moreover, the capacities of ternary solvents [DBUH][4-F-PhO]-4-F-PhOH-EG decrease with increasing concentration of 4-F-PhOH in the solvents. NMR and Fourier transform infrared (FTIR) results demonstrate that CO2 reacts with EG in [DBUH][4-F-PhO]-EG or [DBUH][4-F-PhO]-4-F-PhOH-EG by forming a carbonate species, while [DBUH][4-F-PhO]-4-F-PhOH binary mixtures are chemically inert to CO2. NMR analysis and theoretical calculations evidence that the strength of hydrogen bonds between [4-F-PhO]− and hydrogen-bond donors (EG and 4-F-PhOH) governs the CO2 absorption behaviors, and the strength of the hydrogen bond between the anion [4-F-PhO]− and 4-F-PhOH is much stronger than that between [4-F-PhO]− and EG. Moreover, the desorption behaviors of the DESs studied can also be controlled by tuning the strength of the hydrogen bonds in the solvents. This work highlights the important role of hydrogen bonds in CO2 capture, which may be useful for the rational design of efficient solvents for carbon capture in the future.
中文翻译:

氢键对 4-氟苯酚衍生的功能化低共熔溶剂捕获 CO2 的影响
由于其独特的性质,低共熔溶剂 (DES) 在吸收CO 2方面受到了广泛关注。在此,研究了基于 4-氟苯酚衍生的超碱离子液体的低共熔溶剂 (DES) 用于 CO 2捕获。使用的离子液体是 [DBUH][4-F-PhO],由 1,8-二氮杂双环[5.4.0]十一烷-7-烯 (DBU) 和 4-氟苯酚 (4-F-PhOH) 形成。DES 是通过将 [DBUH][4-F-PhO] 与乙二醇 (EG) 或 4-F-PhOH 混合获得的。令人惊讶的是,[DBUH][ 4 -F-PhO]-EG DESs比 [DBUH][4-F-PhO]-4-F- PhOH ( ∼0.10 摩尔 CO 2/mol 溶剂) 在 25 °C 和 1.0 atm。然而,将EG加入[DBUH][4-F-PhO]-4-F-PhOH后,三元溶剂[DBUH][4-F-PhO]-4-F-PhOH-EG表现出意想不到的高容量, 尽管 EG 和 [DBUH][4-F-PhO]-4-F-PhOH 都表现出低容量。此外,三元溶剂 [DBUH][4-F-PhO]-4-F-PhOH-EG 的容量随着溶剂中 4-F-PhOH 浓度的增加而降低。NMR 和傅里叶变换红外 (FTIR) 结果表明,CO 2在 [DBUH][4-F-PhO]-EG 或 [DBUH][4-F-PhO]-4-F-PhOH-EG 中与 EG 反应,形成一种碳酸盐物质,而 [DBUH][4-F-PhO]-4-F-PhOH 二元混合物对 CO 2具有化学惰性。NMR 分析和理论计算证明 [4-F-PhO] 之间的氢键强度-和氢键供体(EG和4-F-PhOH)控制CO 2吸收行为,并且阴离子[4-F-PhO] -和4-F-PhOH之间的氢键强度比它强得多在 [4-F-PhO] -和 EG 之间。此外,所研究的 DES 的解吸行为也可以通过调节溶剂中氢键的强度来控制。这项工作突出了氢键在CO 2捕获中的重要作用,这可能对未来合理设计用于碳捕获的高效溶剂有用。
更新日期:2023-04-07
中文翻译:

氢键对 4-氟苯酚衍生的功能化低共熔溶剂捕获 CO2 的影响
由于其独特的性质,低共熔溶剂 (DES) 在吸收CO 2方面受到了广泛关注。在此,研究了基于 4-氟苯酚衍生的超碱离子液体的低共熔溶剂 (DES) 用于 CO 2捕获。使用的离子液体是 [DBUH][4-F-PhO],由 1,8-二氮杂双环[5.4.0]十一烷-7-烯 (DBU) 和 4-氟苯酚 (4-F-PhOH) 形成。DES 是通过将 [DBUH][4-F-PhO] 与乙二醇 (EG) 或 4-F-PhOH 混合获得的。令人惊讶的是,[DBUH][ 4 -F-PhO]-EG DESs比 [DBUH][4-F-PhO]-4-F- PhOH ( ∼0.10 摩尔 CO 2/mol 溶剂) 在 25 °C 和 1.0 atm。然而,将EG加入[DBUH][4-F-PhO]-4-F-PhOH后,三元溶剂[DBUH][4-F-PhO]-4-F-PhOH-EG表现出意想不到的高容量, 尽管 EG 和 [DBUH][4-F-PhO]-4-F-PhOH 都表现出低容量。此外,三元溶剂 [DBUH][4-F-PhO]-4-F-PhOH-EG 的容量随着溶剂中 4-F-PhOH 浓度的增加而降低。NMR 和傅里叶变换红外 (FTIR) 结果表明,CO 2在 [DBUH][4-F-PhO]-EG 或 [DBUH][4-F-PhO]-4-F-PhOH-EG 中与 EG 反应,形成一种碳酸盐物质,而 [DBUH][4-F-PhO]-4-F-PhOH 二元混合物对 CO 2具有化学惰性。NMR 分析和理论计算证明 [4-F-PhO] 之间的氢键强度-和氢键供体(EG和4-F-PhOH)控制CO 2吸收行为,并且阴离子[4-F-PhO] -和4-F-PhOH之间的氢键强度比它强得多在 [4-F-PhO] -和 EG 之间。此外,所研究的 DES 的解吸行为也可以通过调节溶剂中氢键的强度来控制。这项工作突出了氢键在CO 2捕获中的重要作用,这可能对未来合理设计用于碳捕获的高效溶剂有用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号