当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Cage Walking” Synthetic Strategy for Unusual Unsymmetrical Supramolecular Cages
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-06 , DOI: 10.1021/jacs.3c00866 Xin-Ran Liu 1 , Peng-Fei Cui 1 , Shu-Ting Guo 1 , Yue-Jian Lin 1 , Guo-Xin Jin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-06 , DOI: 10.1021/jacs.3c00866 Xin-Ran Liu 1 , Peng-Fei Cui 1 , Shu-Ting Guo 1 , Yue-Jian Lin 1 , Guo-Xin Jin 1
Affiliation
|
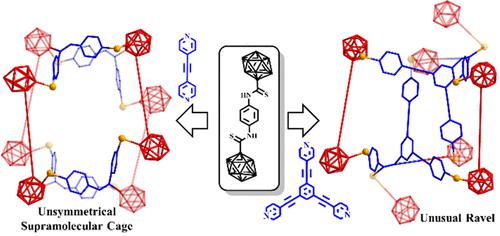
Developing novel assembly methods for supramolecular compounds has long been a research challenge. Herein, we describe how to integrate the B–C coupling reaction and “cage walking” process into coordination self-assembly to construct supramolecular cages. In this strategy, dipyridine linkers containing alkynes react with the metallized carborane backbone through B–C coupling and then “cage walking” resulting in metallacages. However, dipyridine linkers without alkynyl groups can form only metallacycles. We can regulate the size of metallacages based on the length of the alkynyl bipyridine linkers. When tridentate-pyridine linkers participate in this reaction, a new type of ravel is formed. The metallization of carboranes, the B–C coupling reaction, and especially the “cage walking” process of carborane cages play a vital role in this reaction. This work provides a promising principle for the synthesis of metallacages and opens up a novel opportunity in the supramolecular field.
中文翻译:

异常不对称超分子笼的“笼走”合成策略
长期以来,开发超分子化合物的新型组装方法一直是一项研究挑战。在此,我们描述了如何将 B-C 偶联反应和“笼行走”过程整合到协调自组装中以构建超分子笼。在该策略中,含有炔烃的联吡啶接头通过 B-C 偶联与金属化碳硼烷主链反应,然后“笼走”产生金属笼。然而,没有炔基的联吡啶接头只能形成金属环。我们可以根据炔基联吡啶接头的长度来调节金属笼的大小。当三齿-吡啶接头参与该反应时,会形成一种新型的拉环。碳硼烷的金属化、B-C偶联反应,尤其是碳硼烷笼的“笼走”过程在该反应中起着至关重要的作用。
更新日期:2023-04-06
中文翻译:

异常不对称超分子笼的“笼走”合成策略
长期以来,开发超分子化合物的新型组装方法一直是一项研究挑战。在此,我们描述了如何将 B-C 偶联反应和“笼行走”过程整合到协调自组装中以构建超分子笼。在该策略中,含有炔烃的联吡啶接头通过 B-C 偶联与金属化碳硼烷主链反应,然后“笼走”产生金属笼。然而,没有炔基的联吡啶接头只能形成金属环。我们可以根据炔基联吡啶接头的长度来调节金属笼的大小。当三齿-吡啶接头参与该反应时,会形成一种新型的拉环。碳硼烷的金属化、B-C偶联反应,尤其是碳硼烷笼的“笼走”过程在该反应中起着至关重要的作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号