当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binary Mixtures of n-Tridecane with n-Alkylcyclohexanes: Density, Viscosity, and Speed of Sound within the Temperature Range (288.15 to 333.15) K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-04-05 , DOI: 10.1021/acs.jced.2c00772 Dianne Jeanne Luning Prak 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-04-05 , DOI: 10.1021/acs.jced.2c00772 Dianne Jeanne Luning Prak 1
Affiliation
|
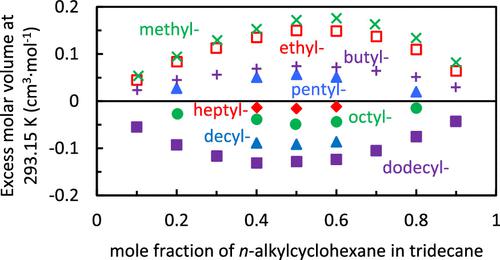
Measurements of the densities, viscosities, and speeds of sound of binary mixtures of n-tridecane and n-alkylcyclohexanes (methyl-, ethyl-, butyl-, pentyl-, heptyl-, octyl-, decyl-, and dodecylcyclohexanes) are reported at various mole fractions. Mixture densities, viscosities, and speeds of sound increased with a decrease in temperature and an increase in the component with the higher property value. Excess molar volumes (VmE’s) decreased with increasing alkyl chain length on the n-alkylcyclohexanes. The excess speeds of sound (cE’s) decreased as the n-alkyl chain length decreased until a minimum was reached for ethylcyclohexane, and then it increased for methylcyclohexane. For all molecules tested except methylcyclohexane, VmE’s and excess isentropic compressibilities (KsE’s) had the same sign, suggesting that the amount of space taken up by the molecules was influencing compressibility. Methylcyclohexane, however, had the largest positive VmE, but its excess compressibility was negative and close to zero. Its extra space was not more compressible. The VmE’s, cE’s, and viscosity deviations for n-tridecane mixtures fell between those reported for n-dodecane and n-hexadecane. These data and trends can be used by fuel researchers who are formulating mixtures to represent various fuels.
中文翻译:

正十三烷与正烷基环己烷的二元混合物:温度范围(288.15 至 333.15)K 内的密度、粘度和声速
正十三烷和正烷基环己烷(甲基-、乙基-、丁基-、戊基-、庚基-、辛基-、癸基-和十二烷基环己烷)的二元混合物的密度、粘度和声速的测量报告在各种摩尔分数。混合物密度、粘度和声速随着温度的降低和具有较高属性值的组分的增加而增加。过量摩尔体积 ( V m E ’s) 随着正烷基环己烷上烷基链长度的增加而减少。超音速 ( c E ’s) 随着n-烷基链长度减少,直到乙基环己烷达到最小值,然后甲基环己烷增加。对于除甲基环己烷之外的所有测试分子,V m E和过量等熵压缩率 ( K s E ) 具有相同的符号,这表明分子占据的空间量正在影响可压缩性。然而,甲基环己烷具有最大的正V m E,但其过压系数为负且接近于零。它的额外空间不是更可压缩的。n的 V m E ’ s、c E ’s 和粘度偏差-十三烷混合物介于报告的正十二烷和正十六烷之间。这些数据和趋势可供正在配制混合物以代表各种燃料的燃料研究人员使用。
更新日期:2023-04-05
中文翻译:

正十三烷与正烷基环己烷的二元混合物:温度范围(288.15 至 333.15)K 内的密度、粘度和声速
正十三烷和正烷基环己烷(甲基-、乙基-、丁基-、戊基-、庚基-、辛基-、癸基-和十二烷基环己烷)的二元混合物的密度、粘度和声速的测量报告在各种摩尔分数。混合物密度、粘度和声速随着温度的降低和具有较高属性值的组分的增加而增加。过量摩尔体积 ( V m E ’s) 随着正烷基环己烷上烷基链长度的增加而减少。超音速 ( c E ’s) 随着n-烷基链长度减少,直到乙基环己烷达到最小值,然后甲基环己烷增加。对于除甲基环己烷之外的所有测试分子,V m E和过量等熵压缩率 ( K s E ) 具有相同的符号,这表明分子占据的空间量正在影响可压缩性。然而,甲基环己烷具有最大的正V m E,但其过压系数为负且接近于零。它的额外空间不是更可压缩的。n的 V m E ’ s、c E ’s 和粘度偏差-十三烷混合物介于报告的正十二烷和正十六烷之间。这些数据和趋势可供正在配制混合物以代表各种燃料的燃料研究人员使用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号