当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-Allylhydroxyamine: A Bifunctional Olefin for Construction of Axially and Centrally Chiral Amino Alcohols via Asymmetric Carboamidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-04 , DOI: 10.1021/jacs.3c01162 Ruijie Mi 1 , Zhiying Ding 1 , Songjie Yu 2, 3 , Robert H Crabtree 4 , Xingwei Li 1, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-04 , DOI: 10.1021/jacs.3c01162 Ruijie Mi 1 , Zhiying Ding 1 , Songjie Yu 2, 3 , Robert H Crabtree 4 , Xingwei Li 1, 5
Affiliation
|
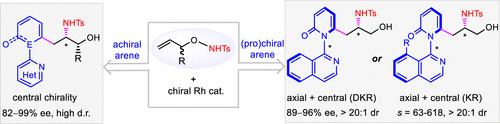
Difunctionalization of olefins offers an attractive approach to access complex chiral structures. Reported herein is the design of N-protected O-allylhydroxyamines as bifunctional olefins that undergo catalytic asymmetric 1,2-carboamidation with three classes of (hetero)arenes to afford chiral amino alcohols via C–H activation. The C═C bond in O-allylhydroxyamine is activated by the intramolecular electrophilic amidating moiety as well as a migrating directing group. The asymmetric carboamidation reaction pattern depends on the nature of the (hetero)arene reagent. Simple achiral (hetero)arenes reacted to give centrally chiral β-amino alcohols in excellent enantioselectivity. The employment of axially prochiral or axially racemic heteroarenes afforded amino alcohols with both axial and central chirality in excellent enantio- and diastereoselectivity. In the case of axially racemic heteroarenes, the coupling follows a kinetic resolution pattern with an s-factor of up to >600. A nitrene-based reaction mechanism has been suggested based on experimental studies, and a unique mode of induction of enantio- and diastereoselectivity has been proposed. Applications of the amino alcohol products have been demonstrated.
中文翻译:

O-烯丙基羟胺:用于通过不对称碳酰胺化构建轴向和中心手性氨基醇的双功能烯烃
烯烃的双官能化提供了一种获得复杂手性结构的有吸引力的方法。本文报道了N-保护的O-烯丙基羟基胺作为双功能烯烃的设计,这些烯烃与三类(杂)芳烃进行催化不对称 1,2-碳酰胺化反应,通过 C-H 活化得到手性氨基醇。O中的C═C键-烯丙基羟胺被分子内亲电酰胺化部分以及迁移导向基团激活。不对称碳酰胺化反应模式取决于(杂)芳烃试剂的性质。简单的非手性(杂)芳烃反应生成具有优异对映选择性的中心手性 β-氨基醇。轴向前手性或轴向外消旋杂芳烃的使用提供了具有轴向和中心手性的氨基醇,具有出色的对映和非对映选择性。在轴向外消旋杂芳烃的情况下,偶联遵循具有s 的动力学拆分模式-高达 >600 的系数。基于实验研究提出了一种基于氮烯的反应机理,并提出了一种独特的对映和非对映选择性诱导模式。氨基醇产品的应用已经得到证明。
更新日期:2023-04-04
中文翻译:

O-烯丙基羟胺:用于通过不对称碳酰胺化构建轴向和中心手性氨基醇的双功能烯烃
烯烃的双官能化提供了一种获得复杂手性结构的有吸引力的方法。本文报道了N-保护的O-烯丙基羟基胺作为双功能烯烃的设计,这些烯烃与三类(杂)芳烃进行催化不对称 1,2-碳酰胺化反应,通过 C-H 活化得到手性氨基醇。O中的C═C键-烯丙基羟胺被分子内亲电酰胺化部分以及迁移导向基团激活。不对称碳酰胺化反应模式取决于(杂)芳烃试剂的性质。简单的非手性(杂)芳烃反应生成具有优异对映选择性的中心手性 β-氨基醇。轴向前手性或轴向外消旋杂芳烃的使用提供了具有轴向和中心手性的氨基醇,具有出色的对映和非对映选择性。在轴向外消旋杂芳烃的情况下,偶联遵循具有s 的动力学拆分模式-高达 >600 的系数。基于实验研究提出了一种基于氮烯的反应机理,并提出了一种独特的对映和非对映选择性诱导模式。氨基醇产品的应用已经得到证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号