当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploration of a new class of monoamine oxidase B inhibitors by assembling benzyloxy pharmacophore on halogenated chalcones
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-04-03 , DOI: 10.1111/cbdd.14238
Ashutosh Kumar Singh 1 , Seong-Min Kim 2 , Jong Min Oh 2 , Mohamed A Abdelgawad 3, 4 , Mohammed M Ghoneim 5 , T M Rangarajan 6 , Sunil Kumar 1 , Sachithra Thazhathuveedu Sudevan 1 , Daniela Trisciuzzi 7 , Orazio Nicolotti 7 , Hoon Kim 2 , Bijo Mathew 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-04-03 , DOI: 10.1111/cbdd.14238
Ashutosh Kumar Singh 1 , Seong-Min Kim 2 , Jong Min Oh 2 , Mohamed A Abdelgawad 3, 4 , Mohammed M Ghoneim 5 , T M Rangarajan 6 , Sunil Kumar 1 , Sachithra Thazhathuveedu Sudevan 1 , Daniela Trisciuzzi 7 , Orazio Nicolotti 7 , Hoon Kim 2 , Bijo Mathew 1
Affiliation
|
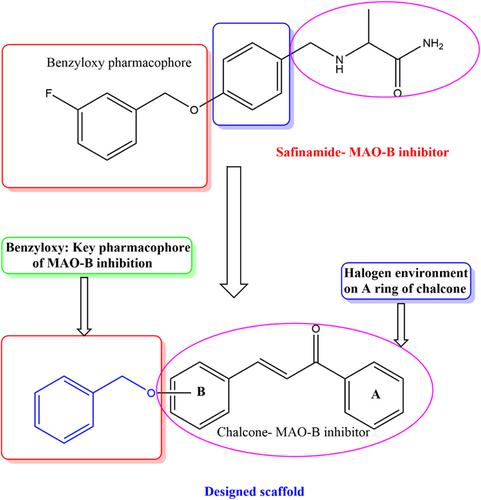
Eight derivatives of benzyloxy-derived halogenated chalcones (BB1-BB8) were synthesized and tested for their ability to inhibit monoamine oxidases (MAOs). MAO-A was less efficiently inhibited by all compounds than MAO-B. Additionally, the majority of the compounds displayed significant MAO-B inhibitory activities at 1 μM with residual activities of less than 50%. With an IC50 value of 0.062 μM, compound BB4 was the most effective in inhibiting MAO-B, followed by compound BB2 (IC50 = 0.093 μM). The lead molecules showed good activity than the reference MAO-B inhibitors (Lazabemide IC50 = 0.11 μM and Pargyline Pargyline IC50 = 0.14). The high selectivity index (SI) values for MAO-B were observed in compounds BB2 and BB4 (430.108 and 645.161, respectively). Kinetics and reversibility experiments revealed that BB2 and BB4 were reversible competitive MAO-B inhibitors with Ki values of 0.030 ± 0.014 and 0.011 ± 0.005 μM, respectively. Swiss target prediction confirmed the high probability in the targets of MAO-B for both compounds. Hypothetical binding mode revealed that the BB2 or BB4 is similarly oriented to the binding cavity of MAO-B. Based on the modelling results, BB4 showed a stable confirmation during the dynamic simulation. From these results, it was concluded that BB2 and BB4 were potent selective reversible MAO-B inhibitors and they can be considered drug candidates for treating related neurodegenerative diseases such as Parkinson's disease.
中文翻译:

通过在卤代查耳酮上组装苄氧基药效团探索一类新型单胺氧化酶 B 抑制剂
合成了八种苄氧基衍生的卤代查耳酮衍生物 ( BB1-BB8 ),并测试了它们抑制单胺氧化酶 (MAO) 的能力。所有化合物对 MAO-A 的抑制效率均低于 MAO-B。此外,大多数化合物在 1 μM 时表现出显着的 MAO-B 抑制活性,残留活性低于 50%。化合物BB4抑制MAO-B的IC 50值为0.062 μM ,其次是化合物BB2 (IC 50 = 0.093 μM)。先导分子比参考 MAO-B 抑制剂(Lazabemide IC 50 = 0.11 μM 和 Pargyline Pargyline IC 50 = 0.14)表现出良好的活性。在化合物BB2和BB4中观察到 MAO-B 的高选择性指数 (SI) 值(分别为 430.108 和 645.161)。动力学和可逆性实验表明,BB2和BB4是可逆竞争性 MAO-B 抑制剂,K i值分别为 0.030 ± 0.014 和 0.011 ± 0.005 μM。Swiss 目标预测证实了两种化合物的 MAO-B 目标的高概率。假设的结合模式表明BB2或BB4与MAO-B 的结合腔具有相似的取向。根据建模结果,BB4在动态模拟过程中表现出稳定的确认。从这些结果得出结论,BB2和BB4是有效的选择性可逆MAO-B抑制剂,可被视为治疗帕金森病等相关神经退行性疾病的候选药物。
更新日期:2023-04-03
中文翻译:

通过在卤代查耳酮上组装苄氧基药效团探索一类新型单胺氧化酶 B 抑制剂
合成了八种苄氧基衍生的卤代查耳酮衍生物 ( BB1-BB8 ),并测试了它们抑制单胺氧化酶 (MAO) 的能力。所有化合物对 MAO-A 的抑制效率均低于 MAO-B。此外,大多数化合物在 1 μM 时表现出显着的 MAO-B 抑制活性,残留活性低于 50%。化合物BB4抑制MAO-B的IC 50值为0.062 μM ,其次是化合物BB2 (IC 50 = 0.093 μM)。先导分子比参考 MAO-B 抑制剂(Lazabemide IC 50 = 0.11 μM 和 Pargyline Pargyline IC 50 = 0.14)表现出良好的活性。在化合物BB2和BB4中观察到 MAO-B 的高选择性指数 (SI) 值(分别为 430.108 和 645.161)。动力学和可逆性实验表明,BB2和BB4是可逆竞争性 MAO-B 抑制剂,K i值分别为 0.030 ± 0.014 和 0.011 ± 0.005 μM。Swiss 目标预测证实了两种化合物的 MAO-B 目标的高概率。假设的结合模式表明BB2或BB4与MAO-B 的结合腔具有相似的取向。根据建模结果,BB4在动态模拟过程中表现出稳定的确认。从这些结果得出结论,BB2和BB4是有效的选择性可逆MAO-B抑制剂,可被视为治疗帕金森病等相关神经退行性疾病的候选药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号