Carbon ( IF 10.5 ) Pub Date : 2023-04-01 , DOI: 10.1016/j.carbon.2023.03.065 Anjana Tripathi , Ranjit Thapa
|
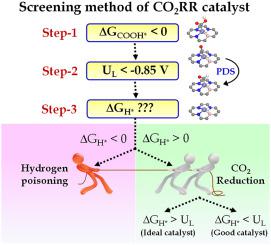
The electrocatalytic reduction of CO2 (CO2RR) into value-added hydrocarbons is limited due to high limiting potential (UL) and competing hydrogen evolution reaction (HER). To find the best catalyst for CO2 reduction the concept of hydrogen poisoning was not considered in the catalyst screening process. Herein, we present a simple screening method and graphical construction using multiparameter optimization for the design of highly active and selective single-atom catalysts (SAC) using density functional theory calculations. A series of SAC namely, MN4, MBN3 and H@MBN3 (M: metal) are investigated for CO2RR. Our results revealed that MN4 and MBN3 SAC are not favorable for CO2RR due to high UL > −0.85 V and hydrogen poisoning (ΔGH* < 0), respectively. H@MBN3 SAC (stable compounds forming H–B bonds) are identified as efficient catalysts with a low value of UL and significantly hinder the competitive HER. Among these, H@CoBN3 and H@FeBN3 SAC show excellent CO2RR activity with limiting potential −0.30 and −0.44 V respectively for CH4 production and no chance of HER. Scaling relations reveal the importance of *COOH/*CHO binding energy (Eb) as an energy descriptor to evaluate the catalytic performance. This work provides a new theoretical perspective to design a highly selective catalyst for CO2RR.
中文翻译:

使用能量描述符的图形构造和识别优化单原子催化剂上的 CO2RR 选择性
由于高极限电位 (U L ) 和竞争性析氢反应 (HER),将 CO 2 (CO 2 RR)电催化还原成增值碳氢化合物受到限制。为了找到用于CO 2还原的最佳催化剂,在催化剂筛选过程中没有考虑氢中毒的概念。在此,我们提出了一种使用多参数优化的简单筛选方法和图形构造,用于使用密度泛函理论计算设计高活性和选择性单原子催化剂 (SAC)。研究了一系列 SAC,即 MN4、MBN3 和 H@MBN3(M:金属)对 CO 2 RR 的影响。我们的结果表明 MN4 和 MBN3 SAC 不利于 CO 2RR 分别由于高 U L > −0.85 V 和氢中毒 (ΔG H* < 0)。H@MBN3 SAC(形成 H-B 键的稳定化合物)被认为是具有低 U L值的高效催化剂,并且显着阻碍了竞争性 HER。其中,H@CoBN3 和 H@FeBN3 SAC 显示出优异的 CO 2 RR 活性,对于 CH 4生产的限制电位分别为 -0.30 和 -0.44 V,并且没有 HER 的机会。比例关系揭示了*COOH/*CHO 结合能 (E b ) 作为评估催化性能的能量描述符的重要性。该工作为设计高选择性CO 2 RR催化剂提供了新的理论视角。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号