当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multi-Catcher Polymers Regulate the Nucleolin Cluster on the Cell Surface for Cancer Therapy
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2023-03-29 , DOI: 10.1002/adhm.202300102
Feng Cheng 1 , Yongjian Jiang 1 , Bo Kong 1 , Huarong Lin 1 , Xinjia Shuai 1 , Pingping Hu 2 , Pengfei Gao 1 , Lei Zhan 1 , Chengzhi Huang 1 , Chunmei Li 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2023-03-29 , DOI: 10.1002/adhm.202300102
Feng Cheng 1 , Yongjian Jiang 1 , Bo Kong 1 , Huarong Lin 1 , Xinjia Shuai 1 , Pingping Hu 2 , Pengfei Gao 1 , Lei Zhan 1 , Chengzhi Huang 1 , Chunmei Li 1
Affiliation
|
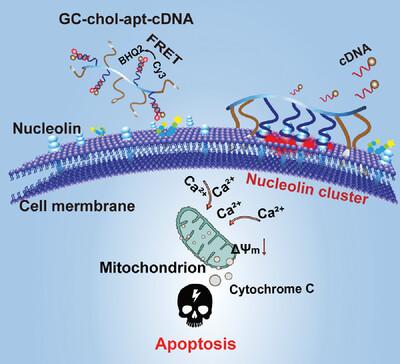
Cell signal transduction mediated by cell surface ligand-receptor is crucial for regulating cell behavior. The oligomerization or hetero-aggregation of the membrane receptor driven by the ligand realizes the rearrangement of apoptotic signals, providing a new ideal tool for tumor therapy. However, the construction of a stable model of cytomembrane receptor aggregation and the development of a universal anti-tumor therapy model on the cellular surface remain challenging. This work describes the construction of a “multi-catcher” flexible structure GC-chol-apt-cDNA with a suitable integration of the oligonucleotide aptamer (apt) and cholesterol (chol) on a polymer skeleton glycol chitosan (GC), for the regulation of the nucleolin cluster through strong polyvalent binding and hydrophobic membrane anchoring on the cell surface. This oligonucleotide aptamer shows nearly 100-fold higher affinity than that of the monovalent aptamer and achieves stable anchoring to the plasma membrane for up to 6 h. Moreover, it exerts a high tumor inhibition both in vitro and in vivo by activating endogenous mitochondrial apoptosis pathway through the cluster of nucleolins on the cell membrane. This multi-catcher nano-platform combines the spatial location regulation of cytomembrane receptors with the intracellular apoptotic signaling cascade and represents a promising strategy for antitumor therapy.
中文翻译:

多捕手聚合物调节细胞表面的核仁素簇用于癌症治疗
由细胞表面配体受体介导的细胞信号转导对于调节细胞行为至关重要。配体驱动的膜受体寡聚或异源聚集实现了凋亡信号的重排,为肿瘤治疗提供了新的理想工具。然而,构建稳定的细胞膜受体聚集模型以及开发细胞表面通用的抗肿瘤治疗模型仍然具有挑战性。这项工作描述了“多捕手”柔性结构GC-chol-apt-cDNA的构建,将寡核苷酸适体(apt)和胆固醇(chol)适当整合在聚合物骨架乙二醇壳聚糖(GC)上,用于调节通过强多价结合和锚定在细胞表面上的疏水膜来形成核仁素簇。该寡核苷酸适体的亲和力比单价适体高近100倍,并可在质膜上稳定锚定长达6小时。此外,它通过细胞膜上的核仁素簇激活内源性线粒体凋亡途径,在体外和体内发挥高肿瘤抑制作用。这种多捕集器纳米平台将细胞膜受体的空间位置调节与细胞内凋亡信号级联相结合,代表了一种有前途的抗肿瘤治疗策略。
更新日期:2023-03-29
中文翻译:

多捕手聚合物调节细胞表面的核仁素簇用于癌症治疗
由细胞表面配体受体介导的细胞信号转导对于调节细胞行为至关重要。配体驱动的膜受体寡聚或异源聚集实现了凋亡信号的重排,为肿瘤治疗提供了新的理想工具。然而,构建稳定的细胞膜受体聚集模型以及开发细胞表面通用的抗肿瘤治疗模型仍然具有挑战性。这项工作描述了“多捕手”柔性结构GC-chol-apt-cDNA的构建,将寡核苷酸适体(apt)和胆固醇(chol)适当整合在聚合物骨架乙二醇壳聚糖(GC)上,用于调节通过强多价结合和锚定在细胞表面上的疏水膜来形成核仁素簇。该寡核苷酸适体的亲和力比单价适体高近100倍,并可在质膜上稳定锚定长达6小时。此外,它通过细胞膜上的核仁素簇激活内源性线粒体凋亡途径,在体外和体内发挥高肿瘤抑制作用。这种多捕集器纳米平台将细胞膜受体的空间位置调节与细胞内凋亡信号级联相结合,代表了一种有前途的抗肿瘤治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号