当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prussian Blue-Derived Nanoplatform for In Situ Amplified Photothermal/Chemodynamic/Starvation Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-03-28 , DOI: 10.1021/acsami.2c22448 Jingyi Liang 1 , Yaning Sun 1 , Kaili Wang 1 , Yawen Zhang 1 , Linqing Guo 1 , Zhihong Bao 1 , Dun Wang 2 , Haiyan Xu 1 , Jiani Zheng 1 , Yue Yuan 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-03-28 , DOI: 10.1021/acsami.2c22448 Jingyi Liang 1 , Yaning Sun 1 , Kaili Wang 1 , Yawen Zhang 1 , Linqing Guo 1 , Zhihong Bao 1 , Dun Wang 2 , Haiyan Xu 1 , Jiani Zheng 1 , Yue Yuan 1
Affiliation
|
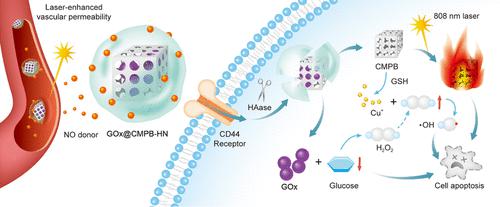
Chemodynamic therapy (CDT) is an emerging tumor treatment; however, it is hindered by insufficient endogenous hydrogen peroxide (H2O2) and high glutathione (GSH) concentrations in the tumor microenvironment (TME). Furthermore, CDT has limited therapeutic efficacy as a monotherapy. To overcome these limitations, in this study, a nanoplatform is designed and constructed from Cu-doped mesoporous Prussian blue (CMPB)-encapsulated glucose oxidase (GOx) with a coating of hyaluronic acid (HA) modified with a nitric oxide donor (HN). In the proposed GOx@CMPB-HN nanoparticles, the dopant Cu2+ ions are crucial to combining and mutually promoting multiple therapeutic approaches, namely, CDT, photothermal therapy (PTT), and starvation therapy. The dopant Cu2+ ions in CMPB protect against reactive oxygen species to deplete the intracellular GSH in the TME. Additionally, the byproduct Cu+ ions act as a substrate for a Fenton-like reaction that activates CDT. Moreover, H2O2, which is another important substrate, is produced in large quantities through intracellular glucose depletion caused by the nanoparticle-loaded GOx, and the gluconic acid produced in this reaction further enhances the TME acidity and creates a better catalytic environment for CDT. In addition, Cu2+ doping greatly improves the mesoporous Prussian blue (MPB) photothermal conversion performance, and the resultant increase in temperature accelerates CDT catalysis. Finally, the HN coating enables the nanoparticles to actively target CD44 receptors in cancer cells and also enhances vascular permeability. Therefore, this coating has multiple effects, such as facilitating enhanced permeability and retention and deep laser penetration. In vitro and in vivo experiments demonstrate that the proposed GOx@CMPB-HN nanoplatform significantly inhibits tumor growth with the help of in situ enhanced synergistic therapies based on the properties of the TME. The developed nanoplatform has the potential to be applied to cancer treatment and introduces new avenues for tumor treatment research.
中文翻译:

用于原位放大光热/化学动力学/饥饿疗法的普鲁士蓝衍生纳米平台
化学动力学疗法(CDT)是一种新兴的肿瘤治疗方法;然而,它受到肿瘤微环境 (TME) 中内源性过氧化氢 (H 2 O 2 ) 不足和高谷胱甘肽 (GSH) 浓度的阻碍。此外,CDT 作为单一疗法的疗效有限。为了克服这些限制,在这项研究中,设计并构建了一个纳米平台,该平台由掺杂铜的介孔普鲁士蓝 (CMPB) 封装的葡萄糖氧化酶 (GOx) 和用一氧化氮供体 (HN) 修饰的透明质酸 (HA) 涂层构成. 在所提出的 GOx@CMPB-HN 纳米粒子中,掺杂剂 Cu 2+离子对于组合和相互促进多种治疗方法至关重要,即 CDT、光热疗法 (PTT) 和饥饿疗法。掺杂剂CuCMPB 中的2+离子可防止活性氧消耗 TME 中的细胞内 GSH。此外,副产物 Cu +离子充当激活 CDT 的类芬顿反应的底物。此外,作为另一个重要底物的H 2 O 2通过纳米颗粒负载的GOx引起的细胞内葡萄糖消耗大量产生,并且该反应中产生的葡萄糖酸进一步增强TME酸度并为TME创造更好的催化环境CDT。此外,Cu 2+掺杂大大提高了介孔普鲁士蓝 (MPB) 光热转换性能,由此产生的温度升高加速了 CDT 催化。最后,HN 涂层使纳米粒子能够主动靶向癌细胞中的 CD44 受体,并增强血管通透性。因此,该涂层具有多种作用,例如促进增强的渗透性和保留以及深度激光穿透。体外和体内实验表明,所提出的 GOx@CMPB-HN 纳米平台借助基于 TME 特性的原位增强协同疗法显着抑制肿瘤生长。所开发的纳米平台具有应用于癌症治疗的潜力,并为肿瘤治疗研究开辟了新的途径。
更新日期:2023-03-28
中文翻译:

用于原位放大光热/化学动力学/饥饿疗法的普鲁士蓝衍生纳米平台
化学动力学疗法(CDT)是一种新兴的肿瘤治疗方法;然而,它受到肿瘤微环境 (TME) 中内源性过氧化氢 (H 2 O 2 ) 不足和高谷胱甘肽 (GSH) 浓度的阻碍。此外,CDT 作为单一疗法的疗效有限。为了克服这些限制,在这项研究中,设计并构建了一个纳米平台,该平台由掺杂铜的介孔普鲁士蓝 (CMPB) 封装的葡萄糖氧化酶 (GOx) 和用一氧化氮供体 (HN) 修饰的透明质酸 (HA) 涂层构成. 在所提出的 GOx@CMPB-HN 纳米粒子中,掺杂剂 Cu 2+离子对于组合和相互促进多种治疗方法至关重要,即 CDT、光热疗法 (PTT) 和饥饿疗法。掺杂剂CuCMPB 中的2+离子可防止活性氧消耗 TME 中的细胞内 GSH。此外,副产物 Cu +离子充当激活 CDT 的类芬顿反应的底物。此外,作为另一个重要底物的H 2 O 2通过纳米颗粒负载的GOx引起的细胞内葡萄糖消耗大量产生,并且该反应中产生的葡萄糖酸进一步增强TME酸度并为TME创造更好的催化环境CDT。此外,Cu 2+掺杂大大提高了介孔普鲁士蓝 (MPB) 光热转换性能,由此产生的温度升高加速了 CDT 催化。最后,HN 涂层使纳米粒子能够主动靶向癌细胞中的 CD44 受体,并增强血管通透性。因此,该涂层具有多种作用,例如促进增强的渗透性和保留以及深度激光穿透。体外和体内实验表明,所提出的 GOx@CMPB-HN 纳米平台借助基于 TME 特性的原位增强协同疗法显着抑制肿瘤生长。所开发的纳米平台具有应用于癌症治疗的潜力,并为肿瘤治疗研究开辟了新的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号