当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Catalytic Interconversion between NADH and NAD+Accompanied by Generation and Consumption of Hydrogen with a Water-Soluble Iridium Complex at Ambient Pressure and Temperature
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-12-16 , DOI: 10.1021/ja207785f
Yuta Maenaka 1 , Tomoyoshi Suenobu 1 , Shunichi Fukuzumi 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-12-16 , DOI: 10.1021/ja207785f
Yuta Maenaka 1 , Tomoyoshi Suenobu 1 , Shunichi Fukuzumi 1, 2
Affiliation
|
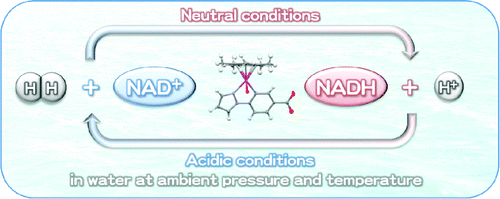
Regioselective hydrogenation of the oxidized form of β-nicotinamide adenine dinucleotide (NAD(+)) to the reduced form (NADH) with hydrogen (H(2)) has successfully been achieved in the presence of a catalytic amount of a [C,N] cyclometalated organoiridium complex [Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))benzoic acid-κC(3))(H(2)O)](2) SO(4) [1](2)·SO(4) under an atmospheric pressure of H(2) at room temperature in weakly basic water. The structure of the corresponding benzoate complex Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))-benzoate-κC(3))(H(2)O) 2 has been revealed by X-ray single-crystal structure analysis. The corresponding iridium hydride complex formed under an atmospheric pressure of H(2) undergoes the 1,4-selective hydrogenation of NAD(+) to form 1,4-NADH. On the other hand, in weakly acidic water the complex 1 was found to catalyze the hydrogen evolution from NADH to produce NAD(+) without photoirradiation at room temperature. NAD(+) exhibited an inhibitory behavior in both catalytic hydrogenation of NAD(+) with H(2) and H(2) evolution from NADH due to the binding of NAD(+) to the catalyst. The overall catalytic mechanism of interconversion between NADH and NAD(+) accompanied by generation and consumption of H(2) was revealed on the basis of the kinetic analysis and detection of the catalytic intermediates.
中文翻译:

在常压和常温下,水溶性铱配合物产生和消耗氢气的 NADH 和 NAD+之间的高效催化互变
β-烟酰胺腺嘌呤二核苷酸(NAD(+))的氧化形式(NAD(+))与氢(H(2))的还原形式(NADH)的区域选择性氢化已在催化量的[C,N ] 环金属化有机铱络合物 [Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))benzoic acid-κC(3))(H(2)O)](2) SO (4) [1](2)·SO(4) 在常压 H(2) 下,室温弱碱性水中。相应的苯甲酸酯络合物 Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))-benzoate-κC(3))(H(2)O) 2 的结构已为通过 X 射线单晶结构分析揭示。在 H(2) 的大气压下形成的相应的氢化铱络合物经历 NAD(+) 的 1,4-选择性氢化以形成 1,4-NADH。另一方面,在弱酸性水中,发现配合物 1 在室温下无需光照射即可催化 NADH 析氢以产生 NAD(+)。由于 NAD(+) 与催化剂的结合,NAD(+) 在 NAD(+) 与 H(2) 和 H(2) 进化的 NAD(+) 催化氢化中表现出抑制行为。NADH 和NAD(+) 之间相互转化的整体催化机制伴随着H(2) 的产生和消耗,是基于催化中间体的动力学分析和检测揭示的。
更新日期:2011-12-16
中文翻译:

在常压和常温下,水溶性铱配合物产生和消耗氢气的 NADH 和 NAD+之间的高效催化互变
β-烟酰胺腺嘌呤二核苷酸(NAD(+))的氧化形式(NAD(+))与氢(H(2))的还原形式(NADH)的区域选择性氢化已在催化量的[C,N ] 环金属化有机铱络合物 [Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))benzoic acid-κC(3))(H(2)O)](2) SO (4) [1](2)·SO(4) 在常压 H(2) 下,室温弱碱性水中。相应的苯甲酸酯络合物 Ir(III)(Cp*)(4-(1H-pyrazol-1-yl-κN(2))-benzoate-κC(3))(H(2)O) 2 的结构已为通过 X 射线单晶结构分析揭示。在 H(2) 的大气压下形成的相应的氢化铱络合物经历 NAD(+) 的 1,4-选择性氢化以形成 1,4-NADH。另一方面,在弱酸性水中,发现配合物 1 在室温下无需光照射即可催化 NADH 析氢以产生 NAD(+)。由于 NAD(+) 与催化剂的结合,NAD(+) 在 NAD(+) 与 H(2) 和 H(2) 进化的 NAD(+) 催化氢化中表现出抑制行为。NADH 和NAD(+) 之间相互转化的整体催化机制伴随着H(2) 的产生和消耗,是基于催化中间体的动力学分析和检测揭示的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号