当前位置:
X-MOL 学术
›
ACS ES&T Water
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Activation of Peroxydisulfate and Hydrogen Peroxide by Sulfidated Nanoscale Zero-Valent Iron for Efficient MTBE Degradation: Significant Role of Oxygen Vacancy
ACS ES&T Water ( IF 4.8 ) Pub Date : 2023-03-27 , DOI: 10.1021/acsestwater.3c00002 Jiaolong Qin 1, 2 , Yan Wei 1 , Wei Geng 1 , Xiaojuan Yu 1 , Baoxue Zhou 1 , Mingce Long 1
ACS ES&T Water ( IF 4.8 ) Pub Date : 2023-03-27 , DOI: 10.1021/acsestwater.3c00002 Jiaolong Qin 1, 2 , Yan Wei 1 , Wei Geng 1 , Xiaojuan Yu 1 , Baoxue Zhou 1 , Mingce Long 1
Affiliation
|
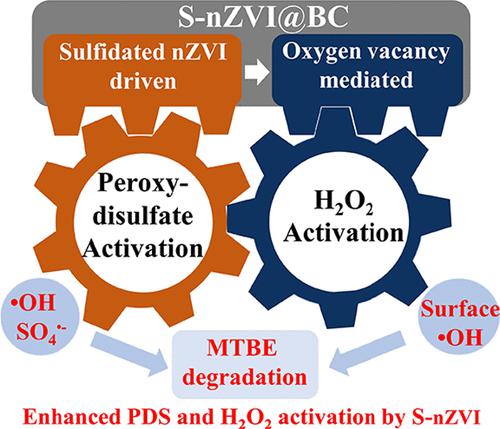
Nanoscale zero-valent iron (nZVI)-based advanced oxidation processes (AOPs) are limited by the rapidly formed surface layer of iron (oxyhydr) oxides. This restriction can be broken by the simultaneous activation of H2O2 and peroxydisulfate (PDS, S2O82–) over sulfidated nanoscale ZVI (S-nZVI), which displayed a synergistic effect to alleviate the drawbacks of the oxidants used alone. In this work, a biochar-supported S-nZVI (noted as S-nZVI@BC) was employed to simultaneously activate PDS and H2O2 for methyl tert-butyl ether (MTBE) degradation, and the rate constant for S-nZVI@BC/Bi-ox (Bi-ox, bi-oxidant at 1:1 molar ratio of PDS and H2O2) was 3.7-, 4.5-, and 12.8-fold higher than that of nZVI@BC/Bi-ox, S-nZVI@BC/PDS, and S-nZVI@BC/H2O2. According to electron paramagnetic resonance (EPR), X-ray photoelectric spectroscopy (XPS), and in-situ oxygen detection analyses, oxygen vacancies were generated over the shell of S-nZVI@BC during PDS activation, and the oxygen vacancy-contained surface layers promoted H2O2 adsorption and dissociation to produce surface-bound ·OH (·OHads), thus significantly improving H2O2 utilization efficiency and accelerating MTBE degradation. These findings provide promising S-nZVI-based AOPs by combining H2O2 and peroxydisulfate activation for environmental remediation and bring insights for the creation of oxygen vacancy-containing materials for peroxide activation.
中文翻译:

硫化纳米级零价铁同时激活过二硫酸盐和过氧化氢以高效降解 MTBE:氧空位的重要作用
基于纳米级零价铁 (nZVI) 的高级氧化工艺 (AOP) 受到快速形成的铁(羟基)氧化物表面层的限制。通过在硫化纳米级 ZVI (S-nZVI) 上同时激活 H 2 O 2和过二硫酸盐 (PDS, S 2 O 8 2– )可以打破这种限制,这显示出协同效应,可以减轻单独使用氧化剂的缺点. 在这项工作中,采用生物炭支持的 S-nZVI(记为 S-nZVI@BC)同时激活 PDS 和 H 2 O 2以降解甲基叔丁基醚 (MTBE),以及 S-nZVI 的速率常数@BC/Bi-ox(Bi-ox,PDS 和 H 2 O摩尔比为 1:1 的双氧化剂2 )比nZVI@BC/Bi-ox、S-nZVI@BC/PDS、S-nZVI@BC/H 2 O 2高3.7、4.5、12.8倍。根据电子顺磁共振 (EPR)、X 射线光电能谱 (XPS) 和原位氧检测分析,在 PDS 活化过程中,S-nZVI@BC 的壳层上产生了氧空位,并且包含氧空位的表面层促进H 2 O 2吸附和解离产生表面结合的· OH(· OH ads),从而显着提高H 2 O 2利用效率和加速 MTBE 降解。这些发现通过将 H 2 O 2和过二硫酸盐活化结合用于环境修复,提供了有前途的基于 S-nZVI 的 AOP ,并为创建用于过氧化物活化的含氧空位材料提供了见解。
更新日期:2023-03-27
中文翻译:

硫化纳米级零价铁同时激活过二硫酸盐和过氧化氢以高效降解 MTBE:氧空位的重要作用
基于纳米级零价铁 (nZVI) 的高级氧化工艺 (AOP) 受到快速形成的铁(羟基)氧化物表面层的限制。通过在硫化纳米级 ZVI (S-nZVI) 上同时激活 H 2 O 2和过二硫酸盐 (PDS, S 2 O 8 2– )可以打破这种限制,这显示出协同效应,可以减轻单独使用氧化剂的缺点. 在这项工作中,采用生物炭支持的 S-nZVI(记为 S-nZVI@BC)同时激活 PDS 和 H 2 O 2以降解甲基叔丁基醚 (MTBE),以及 S-nZVI 的速率常数@BC/Bi-ox(Bi-ox,PDS 和 H 2 O摩尔比为 1:1 的双氧化剂2 )比nZVI@BC/Bi-ox、S-nZVI@BC/PDS、S-nZVI@BC/H 2 O 2高3.7、4.5、12.8倍。根据电子顺磁共振 (EPR)、X 射线光电能谱 (XPS) 和原位氧检测分析,在 PDS 活化过程中,S-nZVI@BC 的壳层上产生了氧空位,并且包含氧空位的表面层促进H 2 O 2吸附和解离产生表面结合的· OH(· OH ads),从而显着提高H 2 O 2利用效率和加速 MTBE 降解。这些发现通过将 H 2 O 2和过二硫酸盐活化结合用于环境修复,提供了有前途的基于 S-nZVI 的 AOP ,并为创建用于过氧化物活化的含氧空位材料提供了见解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号