Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2023-03-28 , DOI: 10.1016/j.bbadis.2023.166701 Limin Liu 1 , Ting Liu 2 , Rui Jia 3 , Lizi Zhang 3 , Zijian Lv 3 , Zhixiong He 3 , Yishan Qu 3 , Shiren Sun 4 , Fadao Tai 3
|
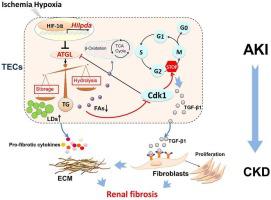
Hypoxia-regulated proximal tubular epithelial cells (PTCs) G2/M phase arrest/delay was involved in production of renal tubulointerstitial fibrosis (TIF). TIF is a common pathological manifestation of progression in patients with chronic kidney disease (CKD), and is often accompanied by lipid accumulation in renal tubules. However, cause-effect relationship between hypoxia-inducible lipid droplet-associated protein (Hilpda), lipid accumulation, G2/M phase arrest/delay and TIF remains unclear. Here we found that overexpression of Hilpda downregulated adipose triglyceride lipase (ATGL) promoted triglyceride overload in the form of lipid accumulation, leading to defective fatty acid β-oxidation (FAO), ATP depletion in a human PTC cell line (HK-2) under hypoxia and in mice kidney tissue treated with unilateral ureteral obstruction (UUO) and unilateral ischemia-reperfusion injury (UIRI). Hilpda-induced lipid accumulation caused mitochondrial dysfunction, enhanced expression of profibrogenic factors TGF-β1, α-SMA and Collagen I elevation, and reduced expression of G2/M phase-associated gene CDK1, as well as increased CyclinB1/D1 ratio, resulted in G2/M phase arrest/delay and profibrogenic phenotypes. Hilpda deficiency in HK-2 cell and kidney of mice with UUO had sustained expression of ATGL and CDK1 and reduced expression of TGF-β1, Collagen I and CyclinB1/D1 ratio, resulting in the amelioration of lipid accumulation and G2/M arrest/delay and subsequent TIF. Expression of Hilpda correlated with lipid accumulation, was positively associated with tubulointerstitial fibrosis in tissue samples from patients with CKD. Our findings suggest that Hilpda deranges fatty acid metabolism in PTCs, which leads to G2/M phase arrest/delay and upregulation of profibrogenic factors, and consequently promote TIF which possibly underlie pathogenesis of CKD.
中文翻译:

Hilpda 导致的脂肪酸氧化下调增加 G2/M 停滞/延迟诱导的肾纤维化
缺氧调节的近端肾小管上皮细胞 (PTC) G2/M 期停滞/延迟参与肾小管间质纤维化 (TIF) 的产生。TIF是慢性肾脏病(chronic kidney disease,CKD)患者病情进展的常见病理表现,常伴有肾小管脂质蓄积。然而,缺氧诱导脂滴相关蛋白 (Hilpda)、脂质积累、G2/M 期阻滞/延迟和 TIF 之间的因果关系仍不清楚。在这里,我们发现 Hilpda 的过表达下调脂肪甘油三酯脂肪酶 (ATGL) 以脂质积累的形式促进甘油三酯过载,导致脂肪酸 β-氧化 (FAO) 缺陷,缺氧条件下人类 PTC 细胞系 (HK-2) 和接受单侧输尿管梗阻 (UUO) 和单侧缺血再灌注损伤 (UIRI) 治疗的小鼠肾组织中的 ATP 耗竭。Hilpda诱导的脂质积累引起线粒体功能障碍,促纤维化因子TGF-β1、α-SMA和I型胶原蛋白表达增强,G2/M期相关基因CDK1表达降低,CyclinB1/D1比值升高,导致G2/M 期停滞/延迟和促纤维化表型。UUO 小鼠 HK-2 细胞和肾脏的 Hilpda 缺陷持续表达 ATGL 和 CDK1,降低 TGF-β1、胶原蛋白 I 和 CyclinB1/D1 比率的表达,从而改善脂质积累和 G2/M 停滞/延迟和随后的 TIF。Hilpda 的表达与脂质积累相关,与 CKD 患者组织样本中的肾小管间质纤维化呈正相关。我们的研究结果表明,Hilpda 会扰乱 PTC 中的脂肪酸代谢,从而导致 G2/M 期停滞/延迟和促纤维化因子的上调,从而促进 TIF,这可能是 CKD 发病机制的基础。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号