当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating the Alkylation Position on Terminal Thiophene Ring of Naphtho[2,3-b:6,7-b′] Bithieno[2,3-d] Thiophene (NBTT) for High-Performance Organic Optoelectronic Devices
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-03-27 , DOI: 10.1021/acsami.3c02547 Zhongwei Liu 1 , Ting Jiang 2 , Yanru Li 1 , Yunpeng Lou 2 , Chan Zhang 1 , Jie Li 2 , Yajing Sun 3, 4 , Xing Chen 1 , Liqiang Li 2, 3 , Hongkun Tian 5 , Deyang Ji 2, 3 , Zhuping Fei 1, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-03-27 , DOI: 10.1021/acsami.3c02547 Zhongwei Liu 1 , Ting Jiang 2 , Yanru Li 1 , Yunpeng Lou 2 , Chan Zhang 1 , Jie Li 2 , Yajing Sun 3, 4 , Xing Chen 1 , Liqiang Li 2, 3 , Hongkun Tian 5 , Deyang Ji 2, 3 , Zhuping Fei 1, 3
Affiliation
|
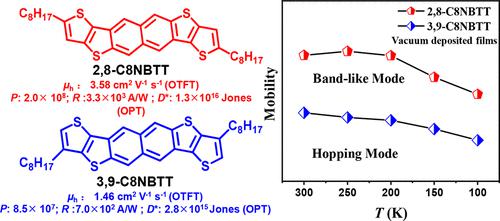
Several thiophene terminated thienoacenes with high mobilities in organic thin-film transistors (OTFTs) have been reported; however, the structure–property relationship of thiophene terminated thienoacenes was unclear, especially the impact of α or β position substitution of terminal thiophene ring on molecular packing and physicochemical properties. Here, we report the synthesis and characterization of a six-ring-fused naphtho[2,3-b:6,7-b′] bithieno[2,3-d] thiophene (NBTT) and its derivatives 2,8-dioctyl-naphtho[2,3-b:6,7-b′] bithieno [2,3-d] thiophene (2,8-C8NBTT) and 3,9-dioctyl-naphtho[2,3-b:6,7-b′] bithieno [2,3-d] thiophene (3,9-C8NBTT). It is found that the alkylation on terminal thiophene ring can effectively tune the molecular stacking from a cofacial herringbone stacking mode (NBTT) to layer-by-layer packing (2,8-C8NBTT and 3,9-C8NBTT). Impressively, a hopping to “band-like” charge transport mechanism evolution of vacuum deposited films is realized by modulating the alkylation position on the terminal thiophene rings. As a result, the OTFTs based on 2,8-C8NBTT characterized by a “band-like” transport presents the highest mobility of 3.58 cm2 V–1 s–1 together with a remarkably high current on/off ratio around 109. Furthermore, organic phototransistors (OPTs) based on 2,8-C8NBTT thin film also exhibits higher photosensitivity (P) of 2.0 × 108, photoresponsivity (R) of 3.3 × 103 A W–1, and detectivity (D*) of 1.3 × 1016 Jones than those based on NBTT and 3,9-C8NBTT.
中文翻译:

调节萘并[2,3-b:6,7-b'] Bithieno[2,3-d] 噻吩 (NBTT) 末端噻吩环上的烷基化位置,用于高性能有机光电器件
已经报道了几种在有机薄膜晶体管 (OTFT) 中具有高迁移率的噻吩封端的噻吩并苯;然而,噻吩末端噻吩并苯的结构-性质关系尚不清楚,特别是末端噻吩环的α或β位取代对分子堆积和理化性质的影响。在这里,我们报告了六环稠合萘并[2,3- b :6,7- b '] bithieno [2,3- d ]噻吩 (NBTT) 及其衍生物 2,8-二辛基的合成和表征-naphtho[2,3- b :6,7- b ′] bithieno [2,3- d ] thiophene (2,8-C8NBTT) 和 3,9-dioctyl-naphtho[2,3- b :6,7 - b ′] 比西诺 [2,3- d] 噻吩 (3,9-C8NBTT)。发现末端噻吩环上的烷基化可以有效地将分子堆叠从共面人字形堆叠模式(NBTT)调整为逐层堆积(2,8-C8NBTT和3,9-C8NBTT)。令人印象深刻的是,通过调节末端噻吩环上的烷基化位置,实现了真空沉积薄膜向“带状”电荷传输机制的跳跃演变。因此,基于以“带状”传输为特征的 2,8-C8NBTT 的 OTFT 具有 3.58 cm 2 V –1 s –1的最高迁移率以及大约 10 9的非常高的电流开/关比。此外,基于2,8-C8NBTT薄膜的有机光电晶体管(OPT)也表现出更高的光敏性(P ) 为 2.0 × 10 8,光响应性 ( R ) 为 3.3 × 10 3 AW –1,探测率 ( D * ) 为 1.3 × 10 16 Jones 比基于 NBTT 和 3,9-C8NBTT 的那些。
更新日期:2023-03-27
中文翻译:

调节萘并[2,3-b:6,7-b'] Bithieno[2,3-d] 噻吩 (NBTT) 末端噻吩环上的烷基化位置,用于高性能有机光电器件
已经报道了几种在有机薄膜晶体管 (OTFT) 中具有高迁移率的噻吩封端的噻吩并苯;然而,噻吩末端噻吩并苯的结构-性质关系尚不清楚,特别是末端噻吩环的α或β位取代对分子堆积和理化性质的影响。在这里,我们报告了六环稠合萘并[2,3- b :6,7- b '] bithieno [2,3- d ]噻吩 (NBTT) 及其衍生物 2,8-二辛基的合成和表征-naphtho[2,3- b :6,7- b ′] bithieno [2,3- d ] thiophene (2,8-C8NBTT) 和 3,9-dioctyl-naphtho[2,3- b :6,7 - b ′] 比西诺 [2,3- d] 噻吩 (3,9-C8NBTT)。发现末端噻吩环上的烷基化可以有效地将分子堆叠从共面人字形堆叠模式(NBTT)调整为逐层堆积(2,8-C8NBTT和3,9-C8NBTT)。令人印象深刻的是,通过调节末端噻吩环上的烷基化位置,实现了真空沉积薄膜向“带状”电荷传输机制的跳跃演变。因此,基于以“带状”传输为特征的 2,8-C8NBTT 的 OTFT 具有 3.58 cm 2 V –1 s –1的最高迁移率以及大约 10 9的非常高的电流开/关比。此外,基于2,8-C8NBTT薄膜的有机光电晶体管(OPT)也表现出更高的光敏性(P ) 为 2.0 × 10 8,光响应性 ( R ) 为 3.3 × 10 3 AW –1,探测率 ( D * ) 为 1.3 × 10 16 Jones 比基于 NBTT 和 3,9-C8NBTT 的那些。































 京公网安备 11010802027423号
京公网安备 11010802027423号