当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Simulation of Menadiol Diacetate Solubility in 14 Solvents at Temperatures from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-03-27 , DOI: 10.1021/acs.jced.2c00734
Xinyi Lü 1 , Xiaotong Yang 1 , Xiaoli Wu 1 , Jidong Wang 1 , Zhong Zhao 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-03-27 , DOI: 10.1021/acs.jced.2c00734
Xinyi Lü 1 , Xiaotong Yang 1 , Xiaoli Wu 1 , Jidong Wang 1 , Zhong Zhao 2
Affiliation
|
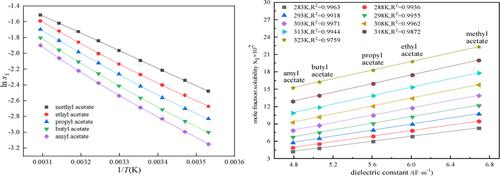
In this paper, the solubility of menadiol diacetate in nine alcohols (methanol, ethanol, n-propanol, i-propanol, n-butanol, i-butanol, n-amyl alcohol, i-amyl alcohol, and cyclohexanol) and five n-alkyl acetates (methyl acetate, ethyl acetate, n-propyl acetate, n-butyl acetate, and amyl acetate) was experimentally measured with the aid of a laser dynamic device at temperatures from 283.15 to 323.15 K. The solubility of menadiol diacetate as a function of temperature was regressed in terms of three semiempirical solubility models (Apelblat, van’t Hoff, and λh), two activity coefficient solubility models (Wilson and NRTL), and the Jouyban model. The thermodynamic mixing properties (mixing Gibbs energy, entropy, and enthalpy) of the solutions were estimated in terms of the measured solubility data and the regressed parameters of the Wilson model.
中文翻译:

283.15 至 323.15 K 温度下甲萘二醇二乙酸酯在 14 种溶剂中溶解度的测定和模拟
本文研究了甲萘二醇二乙酸酯在九种醇类(甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、正戊醇、异戊醇、环己醇)和五种正-乙酸烷基酯(乙酸甲酯、乙酸乙酯、乙酸正丙酯、乙酸正丁酯和乙酸戊酯)在 283.15 至 323.15 K 的温度下借助于激光动态装置进行实验测量。甲萘二醇二乙酸酯的溶解度作为函数温度根据三个半经验溶解度模型(Apelblat、van't Hoff 和 λ h)、两个活度系数溶解度模型(Wilson 和 NRTL)和 Jouyban 模型。根据测得的溶解度数据和 Wilson 模型的回归参数估计溶液的热力学混合特性(混合吉布斯能、熵和焓)。
更新日期:2023-03-27
中文翻译:

283.15 至 323.15 K 温度下甲萘二醇二乙酸酯在 14 种溶剂中溶解度的测定和模拟
本文研究了甲萘二醇二乙酸酯在九种醇类(甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、正戊醇、异戊醇、环己醇)和五种正-乙酸烷基酯(乙酸甲酯、乙酸乙酯、乙酸正丙酯、乙酸正丁酯和乙酸戊酯)在 283.15 至 323.15 K 的温度下借助于激光动态装置进行实验测量。甲萘二醇二乙酸酯的溶解度作为函数温度根据三个半经验溶解度模型(Apelblat、van't Hoff 和 λ h)、两个活度系数溶解度模型(Wilson 和 NRTL)和 Jouyban 模型。根据测得的溶解度数据和 Wilson 模型的回归参数估计溶液的热力学混合特性(混合吉布斯能、熵和焓)。








































 京公网安备 11010802027423号
京公网安备 11010802027423号