The Journal of Prevention of Alzheimer's Disease ( IF 8.5 ) Pub Date : 2023-03-27 , DOI: 10.14283/jpad.2023.30 J Cummings 1 , L Apostolova , G D Rabinovici , A Atri , P Aisen , S Greenberg , S Hendrix , D Selkoe , M Weiner , R C Petersen , S Salloway
|
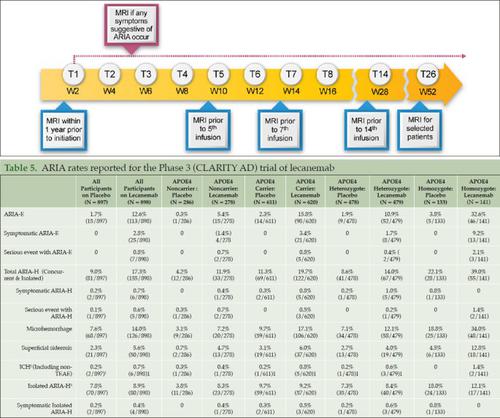
Lecanemab (Leqembi®) is approved in the United States for the treatment of Alzheimer’s disease (AD) to be initiated in early AD (mild cognitive impairment [MCI] due to AD or mild AD dementia) with confirmed brain amyloid pathology. Appropriate Use Recommendations (AURs) are intended to help guide the introduction of new therapies into real-world clinical practice. Community dwelling patients with AD differ from those participating in clinical trials. Administration of lecanemab at clinical trial sites by individuals experienced with monoclonal antibody therapy also differs from the community clinic-based administration of lecanemab. These AURs use clinical trial data as well as research and care information regarding AD to help clinicians administer lecanemab with optimal safety and opportunity for effectiveness. Safety and efficacy of lecanemab are known only for patients like those participating in the phase 2 and phase 3 lecanemab trials, and these AURs adhere closely to the inclusion and exclusion criteria of the trials. Adverse events may occur with lecanemab including amyloid related imaging abnormalities (ARIA) and infusion reactions. Monitoring guidelines for these events are detailed in this AUR. Most ARIA with lecanemab is asymptomatic, but a few cases are serious or, very rarely, fatal. Microhemorrhages and rare macrohemorrhages may occur in patients receiving lecanemab. Anticoagulation increases the risk of hemorrhage, and the AUR recommends that patients requiring anticoagulants not receive lecanemab until more data regarding this interaction are available. Patients who are apolipoprotein E ε4 (APOE4) gene carriers, especially APOE4 homozygotes, are at higher risk for ARIA, and the AUR recommends APOE genotyping to better inform risk discussions with patients who are lecanemab candidates. Clinician and institutional preparedness are mandatory for use of lecanemab, and protocols for management of serious events should be developed and implemented. Communication between clinicians and therapy candidates or those on therapy is a key element of good clinical practice for the use of lecanemab. Patients and their care partners must understand the potential benefits, the potential harms, and the monitoring requirements for treatment with this agent. Culture-specific communication and building of trust between clinicians and patients are the foundation for successful use of lecanemab.
中文翻译:

Lecanemab:适当使用建议
Lecanemab (Leqembi®) 在美国被批准用于治疗阿尔茨海默氏病 (AD),在 AD 早期(AD 引起的轻度认知障碍 [MCI] 或轻度 AD 痴呆)中开始治疗,并已确诊脑淀粉样蛋白病理。适当使用建议 (AUR) 旨在帮助指导将新疗法引入现实临床实践。社区居住的 AD 患者与参加临床试验的患者不同。由具有单克隆抗体治疗经验的个体在临床试验地点给予乐卡奈单抗也不同于基于社区诊所的乐卡奈单抗给药。这些 AUR 使用临床试验数据以及有关 AD 的研究和护理信息来帮助临床医生以最佳的安全性和有效性机会来管理 Lecanemab。 lecanemab 的安全性和有效性仅对于参与 2 期和 3 期 lecanemab 试验的患者而言是已知的,并且这些 AUR 严格遵守试验的纳入和排除标准。 lecanemab 可能会发生不良事件,包括淀粉样蛋白相关成像异常 (ARIA) 和输注反应。本 AUR 中详细介绍了这些事件的监控指南。大多数使用lecanemab 的 ARIA 是无症状的,但也有少数病例很严重,或者在极少数情况下致命。接受 Lecanemab 的患者可能会发生微出血和罕见的大出血。抗凝会增加出血风险,AUR 建议需要抗凝药物的患者在获得有关这种相互作用的更多数据之前不要接受乐卡奈单抗。 载脂蛋白 E ε4 ( APOE4 ) 基因携带者,尤其是APOE4纯合子患者,患 ARIA 的风险较高,AUR 建议进行APOE基因分型,以便更好地与 Lecanemab 候选患者进行风险讨论。临床医生和机构必须做好使用 Lecanemab 的准备,并应制定和实施严重事件的管理方案。临床医生与候选治疗者或接受治疗者之间的沟通是使用 Lecanemab 良好临床实践的关键要素。患者及其护理伙伴必须了解该药物治疗的潜在益处、潜在危害以及监测要求。临床医生和患者之间特定文化的沟通和信任的建立是成功使用lecanemab的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号