Electrochemistry Communications ( IF 4.7 ) Pub Date : 2023-03-26 , DOI: 10.1016/j.elecom.2023.107474 Yuting Liu , Hua Liu , Cheng Wang , Yali Wang , Jiaxing Lu , Huan Wang
|
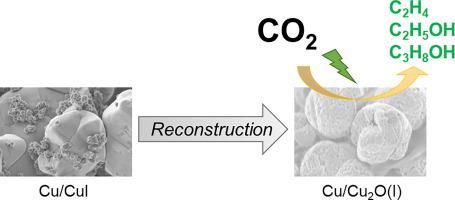
It is particularly desirable for the development of carbon utilization technologies to synthesize multicarbon products (C2+) from the electrochemical CO2 reduction reaction (CO2RR). In this work, Cu/Cu2O(X) catalysts (where X = I, Br, Cl) for selective electroreduction of CO2 to C2+ were reconstructed via cyclic voltammetry on carbon paper. The Faradaic efficiency (FE) for C2+ (C2H4, C2H5OH, C3H8OH) production over Cu/Cu2O(Cl), Cu/Cu2O(Br) and Cu/Cu2O(I) reached 31%, 34% and 58%, respectively, at a potential of −0.76 V vs. the reversible hydrogen electrode (RHE). The Cu+ and Cu0 species obtained from the reconstruction of Cu/CuX are primarily responsible for the high C2+ producing activity.
中文翻译:

用于将 CO2 选择性电还原为 C2+ 产物的重构 Cu/Cu2O(I) 催化剂
从电化学CO 2还原反应(CO 2 RR)合成多碳产物(C 2+ )是碳利用技术发展的特别期望。在这项工作中,通过循环伏安法在复写纸上重建用于将 CO 2选择性电还原为 C 2+的Cu/Cu 2 O(X) 催化剂(其中 X = I、Br、Cl) 。C 2+ (C 2 H 4 , C 2 H 5 OH, C 3 H 8 OH) 在 Cu/Cu 2 O(Cl), Cu/Cu 2 O(Br) 和 Cu/铜相对于可逆氢电极 (RHE),在 -0.76 V 的电位下,2 O(I) 分别达到 31%、34% 和 58%。从 Cu/CuX 的重建中获得的Cu +和 Cu 0物种是产生高 C 2+活性的主要原因。

































 京公网安备 11010802027423号
京公网安备 11010802027423号