Nano Energy ( IF 16.8 ) Pub Date : 2023-03-24 , DOI: 10.1016/j.nanoen.2023.108384 Chunmei Lv , Kai Huang , Yu Fan , Jing Xu , Cheng Lian , Hongliang Jiang , Yongzheng Zhang , Cheng Ma , Wenming Qiao , Jitong Wang , Licheng Ling
|
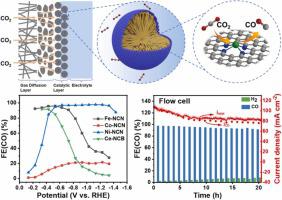
Carbon dioxide electroreduction reaction (CO2RR), as a rational regulation of CO2 resource utilization, demands effectively selective catalysts for converting CO2 into high-value-added chemicals. Carbon-based nanoreactors featuring rationally designed porous framework structures might provide a unique chemical environment for confining and stabilizing the active metal species, consequently improving the CO2RR activity. Herein, nitrogen-doped porous carbon nanospheres decorated by single Ni atom (Ni-NCN) featuring a Ni-N4 structure were synthesized using the modified sol-gel method for the reduction of CO2 to CO. The synergistic effect of the Ni-N4 active sites homogenously distributed in the interconnected pore structure and the favorable chemical confined microspace of carbon nanospheres endows it with excellent CO2RR activity. In the H- type cell, Ni-NCN displays a CO Faradaic efficiency up to 96.6 % and a CO current density of 9.8 mA cm−2 at − 0.83 V (vs. RHE), as well as a high turnover frequency (TOF) of 10658 h−1 at − 1.33 V (vs. RHE). In the flow cell, the mass transfer can be further facilitated by the formation of three-phase interface. The Faradaic efficiency and current density of CO2RR catalyzed by Ni-NCN is enhanced to 97.9 % and 102.4 mA cm−2 at − 1.13 V (vs. RHE), and the wide potential window ranges from − 0.53 V to − 1.33 V (vs. RHE) with the Faradaic efficiency more than 95 %. Density functional theory (DFT) calculations reveal that the high selectivity of Ni-N4 sites is mainly ascribed to the high energy barrier that restrains the hydrogen evolution reaction (HER). Meanwhile, the lower CO binding energy on Ni-N4 site helps the escape of CO to increase the TOF of active sites. The in-situ Fourier transform infrared (FTIR) spectroscopy verifies that the intermediate *COOH can be more stable in the confined environment of Ni-NCN to promote the selectivity of CO2RR. The strategy of constructing confined microspace paves a new path for the rational design of high-efficient single atom catalysts for CO2 reduction.
中文翻译:

利用单镍原子修饰的氮掺杂碳纳米球电催化还原密闭微空间中的二氧化碳
二氧化碳电还原反应(CO 2 RR)作为CO 2资源利用的合理调控,需要有效的选择性催化剂将CO 2转化为高附加值的化学品。具有合理设计的多孔框架结构的碳基纳米反应器可以提供独特的化学环境来限制和稳定活性金属物种,从而提高 CO 2 RR 活性。在此,采用改进的溶胶-凝胶法合成了具有 Ni-N 4结构的单 Ni 原子修饰的氮掺杂多孔碳纳米球 (Ni-NCN),用于将 CO 2还原为 CO。 4 _均匀分布在相互连通的孔结构中的活性位点和碳纳米球有利的化学限制微空间赋予其优异的CO 2 RR活性。在 H 型电池中,Ni-NCN 显示出高达 96.6% 的 CO 法拉第效率和 9.8 mA cm −2的 CO 电流密度(在 − 0.83 V(相对于 RHE))以及高周转频率 (TOF) 10658 h -1在 - 1.33 V(相对于 RHE)。在流通池中,三相界面的形成可以进一步促进传质。Ni-NCN催化CO 2 RR的法拉第效率和电流密度提高到97.9 %和102.4 mA cm -2 at − 1.13 V (vs. RHE), and the wide potential window ranges from − 0.53 V to − 1.33 V (vs. RHE) with the Faradaic efficiency more than 95 %. Density functional theory (DFT) calculations reveal that the high selectivity of Ni-N4 sites is mainly ascribed to the high energy barrier that restrains the hydrogen evolution reaction (HER). Meanwhile, the lower CO binding energy on Ni-N4 site helps the escape of CO to increase the TOF of active sites. The in-situ Fourier transform infrared (FTIR) spectroscopy verifies that the intermediate *COOH can be more stable in the confined environment of Ni-NCN to promote the selectivity of CO2RR。构建受限微空间的策略为合理设计用于CO 2还原的高效单原子催化剂铺平了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号