当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Oxidative [3+2+1] Cyclization of Enaminones and N-Alkenyl-2-pyrrolidinone: Access to Polysubstituted 4-Alkylated 1,4-dihydropyridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-03-25 , DOI: 10.1002/adsc.202300088 Zhangmengjie Chai 1 , Longkun Chen 1 , Zhuoyuan Liu 1 , Yulin Sun 1 , Donghan Liu 1 , Mingshuai Zhang 1 , Yongchao Wang 2 , Fuchao Yu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-03-25 , DOI: 10.1002/adsc.202300088 Zhangmengjie Chai 1 , Longkun Chen 1 , Zhuoyuan Liu 1 , Yulin Sun 1 , Donghan Liu 1 , Mingshuai Zhang 1 , Yongchao Wang 2 , Fuchao Yu 1
Affiliation
|
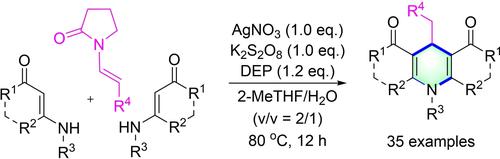
An oxidative [3+2+1] cyclization of enaminones and N-alkenyl-2-pyrrolidinone is described for the synthesis of 4-alkylated 1,4-dihydropyridines (1,4-DHPs). By using terminal olefin as the C4 source of the 1,4-DHP skeleton, this synthetic strategy provides a series of 1,4-DHPs through a 1,1-difunctionalization/cyclization process. In this protocol, two C(sp3)−C(sp2) bonds and a C(sp2)−N bond are simultaneously formed, the hydrogen source on the newly formed methyl group of the 1,4-DHP skeleton is confirmed and a possible mechanism is proposed.
中文翻译:

烯胺酮和 N-烯基-2-吡咯烷酮的氧化 [3+2+1] 环化:获得多取代的 4-烷基化 1,4-二氢吡啶
烯胺酮和N -烯基-2-吡咯烷酮的氧化 [3+2+1] 环化用于合成 4-烷基化 1,4-二氢吡啶 (1,4-DHP)。通过使用末端烯烃作为 1,4-DHP 骨架的 C4 来源,该合成策略通过 1,1-双官能化/环化过程提供了一系列 1,4-DHP。在该方案中,两个C( sp 3 )−C( sp 2 )键和一个C( sp 2 )−N键同时形成,确认了1,4-DHP骨架新形成的甲基上的氢源并提出了一种可能的机制。
更新日期:2023-03-25
中文翻译:

烯胺酮和 N-烯基-2-吡咯烷酮的氧化 [3+2+1] 环化:获得多取代的 4-烷基化 1,4-二氢吡啶
烯胺酮和N -烯基-2-吡咯烷酮的氧化 [3+2+1] 环化用于合成 4-烷基化 1,4-二氢吡啶 (1,4-DHP)。通过使用末端烯烃作为 1,4-DHP 骨架的 C4 来源,该合成策略通过 1,1-双官能化/环化过程提供了一系列 1,4-DHP。在该方案中,两个C( sp 3 )−C( sp 2 )键和一个C( sp 2 )−N键同时形成,确认了1,4-DHP骨架新形成的甲基上的氢源并提出了一种可能的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号