当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Atom Doping Carbon Materials as Highly Efficient Electrocatalysts for Lithium–Sulfur Batteries: Bimetallic Cooperation Mechanism
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.jpcc.2c08262
Tongtong Li 1 , Yangfeng Yu 1 , Mengying Pei 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.jpcc.2c08262
Tongtong Li 1 , Yangfeng Yu 1 , Mengying Pei 1
Affiliation
|
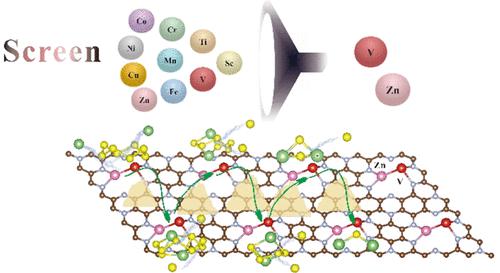
Due to the low sulfur utilization, slow battery kinetics, and shuttle effect of lithium polysulfides (LiPSs), the practical application of lithium–sulfur (Li–S) batteries is severely limited. Understanding the reaction mechanism is very important for the design and application of high-performance batteries. Herein, the adsorption mechanism of LiPSs, the reaction mechanism of a battery electrode, and the catalytic decomposition of LiPSs on pristine, single-atom, and dual-atom doping C9N4 (C9N4, M/C9N4, and M1–M2/C9N4) nanosheets are comprehensively considered for the first time. Through bond length analysis, charge analysis, and energy analysis, the doping of metal atoms, especially co-doping of V and Zn atoms (Zn–V/C9N4), can greatly improve the adsorption performance of material C9N4. More importantly, Zn–V/C9N4 can significantly reduce the reaction energy barrier of the battery electrode (0.144 eV) and the decomposition energy barrier of Li2S (0.661 eV). The simulation results show that high catalytic activity depends on the unique d-orbital coupling and the ″pull″ effect of metal co-doping. These findings are crucial to understanding the role of dual-atom doping carbon materials in the design of cathode materials to cope with the performance constraints in lithium–sulfur batteries. We hope that this research idea can also be applied to other dual-atom systems.
中文翻译:

双原子掺杂碳材料作为锂硫电池的高效电催化剂:双金属合作机制
由于硫利用率低、电池动力学缓慢和多硫化锂(LiPSs)的穿梭效应,锂硫(Li-S)电池的实际应用受到严重限制。了解反应机理对于高性能电池的设计和应用非常重要。在此,LiPSs 的吸附机理、电池电极的反应机理以及 LiPSs 在原始、单原子和双原子掺杂 C 9 N 4 (C 9 N 4 , M / C 9 N 4 )上的催化分解, 和 M 1 –M 2 /C 9 N 4)首次综合考虑了纳米片。通过键长分析、电荷分析和能量分析,金属原子的掺杂,尤其是V和Zn原子的共掺杂(Zn–V/C 9 N 4 ),可以大大提高材料C 9 N 4的吸附性能. 更重要的是,Zn–V/C 9 N 4可以显着降低电池电极的反应能垒(0.144 eV)和Li 2的分解能垒S (0.661 电子伏特)。模拟结果表明,高催化活性取决于独特的 d 轨道耦合和金属共掺杂的“拉”效应。这些发现对于理解双原子掺杂碳材料在阴极材料设计中的作用以应对锂硫电池的性能限制至关重要。我们希望这一研究思路也可以应用于其他双原子系统。
更新日期:2023-03-24
中文翻译:

双原子掺杂碳材料作为锂硫电池的高效电催化剂:双金属合作机制
由于硫利用率低、电池动力学缓慢和多硫化锂(LiPSs)的穿梭效应,锂硫(Li-S)电池的实际应用受到严重限制。了解反应机理对于高性能电池的设计和应用非常重要。在此,LiPSs 的吸附机理、电池电极的反应机理以及 LiPSs 在原始、单原子和双原子掺杂 C 9 N 4 (C 9 N 4 , M / C 9 N 4 )上的催化分解, 和 M 1 –M 2 /C 9 N 4)首次综合考虑了纳米片。通过键长分析、电荷分析和能量分析,金属原子的掺杂,尤其是V和Zn原子的共掺杂(Zn–V/C 9 N 4 ),可以大大提高材料C 9 N 4的吸附性能. 更重要的是,Zn–V/C 9 N 4可以显着降低电池电极的反应能垒(0.144 eV)和Li 2的分解能垒S (0.661 电子伏特)。模拟结果表明,高催化活性取决于独特的 d 轨道耦合和金属共掺杂的“拉”效应。这些发现对于理解双原子掺杂碳材料在阴极材料设计中的作用以应对锂硫电池的性能限制至关重要。我们希望这一研究思路也可以应用于其他双原子系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号