Polyhedron Pub Date : 2023-03-24 , DOI: 10.1016/j.poly.2023.116385
Evgeniya S. Bazhina , Maxim A. Shmelev , Konstantin A. Babeshkin , Nikolay N. Efimov , Mikhail A. Kiskin , Igor L. Eremenko
|
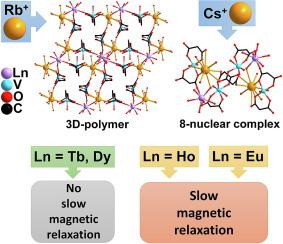
The reactions of oxidovanadium(IV) sulfate with Rb2(cbdc) or Cs2(cbdc) (cbdc2- – dianion of cyclobutane-1,1-dicarboxylic acid) and lanthanide(III) nitrates in water solutions gave two series of heterometallic compounds, namely {[RbLn(VO)2(cbdc)4(H2O)10]·2.5H2O}n (1Ln, Ln = Eu, Tb, Dy, Ho) and {[CsLn(VO)2(cbdc)4(H2O)11]·5H2O}2 (2Ln, Ln = Eu, Tb, Dy, Ho), respectively. The crystal structures of these compounds were determined by X-ray diffraction and were found to be built of similar trinuclear anionic units [Ln(VO)2(cbdc)4(H2O)8]- ({LnV2}-). Due to the structure-forming role of alkali metal ions, the heterometallic {LnV2} cores of these units differ in geometric characteristics (the V···Ln and V···V distances) and spatial arrangement in a crystal. In 1Ln, the {LnV2}- units are bound by rubidium ions into a 3D framework structure, whereas in 2Ln, cesium ions provide the formation of a discrete molecular structure. According to alternating current (ac) magnetic susceptibility measurements, only Eu(III)- and Ho(III)-containing compounds (1Eu, 1Ho, 2Eu, and 2Ho) exhibited field-induced slow relaxation of magnetization.
中文翻译:

由 Rb+ 和 Cs+ 阳离子介导的不同结构类型的两类 Ln(III)-V(IV) 化合物(Ln(III) = Eu、Tb、Dy、Ho):Eu(III)- 和 Ho( III) 含成员
硫酸氧钒 (IV) 与 Rb 2 (cbdc) 或 Cs 2 (cbdc)(cbdc 2- - 环丁烷-1,1-二羧酸的双阴离子)和镧系元素 (III) 硝酸盐在水溶液中的反应得到两个系列的异金属化合物化合物,即 {[RbLn(VO) 2 (cbdc) 4 (H 2 O) 10 ]·2.5H 2 O} n ( 1 Ln , Ln = Eu, Tb, Dy, Ho) 和 {[CsLn(VO) 2 (cbdc) 4 (H 2 O) 11 ]·5H 2 O} 2 ( 2 Ln, Ln = Eu, Tb, Dy, Ho)。这些化合物的晶体结构通过 X 射线衍射确定,并发现它们由相似的三核阴离子单元构成 [Ln(VO) 2 ( cbdc ) 4 (H 2 O) 8 ] - ({LnV 2 } - )。由于碱金属离子的结构形成作用,这些单元的异金属{LnV 2 }核在晶体中的几何特征(V···Ln和V···V距离)和空间排列不同。在1 Ln中,{LnV 2 } -单元被铷离子结合成 3D 框架结构,而在2Ln、铯离子提供了离散分子结构的形成。根据交流电 ( ac ) 磁化率测量,只有含 Eu(III) 和 Ho(III) 的化合物( 1 Eu、 1 Ho、 2 Eu和2 Ho)表现出场诱导的磁化缓慢弛豫。

































 京公网安备 11010802027423号
京公网安备 11010802027423号