当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B(9)-OH-o-Carboranes: Synthesis, Mechanism, and Property Exploration
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-24 , DOI: 10.1021/jacs.2c13570 Yan-Na Ma 1 , Huazhan Ren 2 , Yanxuan Wu 1 , Na Li 2 , Feijing Chen 1 , Xuenian Chen 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-24 , DOI: 10.1021/jacs.2c13570 Yan-Na Ma 1 , Huazhan Ren 2 , Yanxuan Wu 1 , Na Li 2 , Feijing Chen 1 , Xuenian Chen 1, 2
Affiliation
|
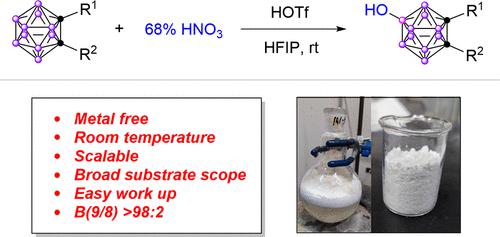
Herein, we present a chemically robust and efficient synthesis route for B(9)-OH-o-carboranes by the oxidation of o-carboranes with commercially available 68% HNO3 under the assistance of trifluoromethanesulfonic acid (HOTf) and hexafluoroisopropanol (HFIP). The reaction is highly efficient with a wide scope of carboranes, and the selectivity of B(9)/B(8) is up to 98:2. The success of this transformation relies on the strong electrophilicity and oxidizability of HNO3, promoted through hydrogen bonds of the Brønsted acid HOTf and the solvent HFIP. Mechanism studies reveal that the oxidation of o-carborane involves an initial electrophilic attack of HNO3 to the hydrogen atom at the most electronegative B(9) of o-carborane. In this transformation, the hydrogen atom of the B–H bond is the nucleophilic site, which is different from the electrophilic substitution reaction, where the boron atom is the nucleophilic site. Therefore, this is an oxidation–reduction reaction of o-carborane under mild conditions in which N(V) → N(III) and H(-I) → H(I). The derivatization of 9-OH-o-carborane was further examined, and the carboranyl group was successfully introduced to an amino acid, polyethylene glycol, biotin, deoxyuridine, and saccharide. Undoubtedly, this approach provides a selective way for the rapid incorporation of carborane moieties into small molecules for application in boron neutron capture therapy, which requires the targeted delivery of boron-rich groups.
中文翻译:

B(9)-OH-o-Carboranes:合成、机理和性质探索
在此,我们通过在三氟甲磺酸 (HOTf) 和六氟异丙醇 (HFIP) 的协助下使用市售的 68% HNO 3氧化邻碳硼烷,提出了一种化学稳健且高效的 B(9)-OH-o-碳硼烷合成路线. 反应效率高,碳硼烷范围广,B(9)/B(8)的选择性高达98:2。这种转化的成功依赖于 HNO 3的强亲电性和氧化性,通过布朗斯台德酸 HOTf 和溶剂 HFIP 的氢键促进。机理研究表明,邻碳硼烷的氧化涉及 HNO 3的初始亲电攻击到邻碳硼烷的最具负电性的 B(9) 处的氢原子。在这种转化中,B-H键的氢原子是亲核位点,不同于亲电取代反应中硼原子是亲核位点。因此,这是邻碳硼烷在温和条件下的氧化还原反应,其中 N(V) → N(III) 和 H(-I) → H(I)。9-OH- o的衍生化-carborane 进一步检查,carboranyl 基团被成功地引入氨基酸、聚乙二醇、生物素、脱氧尿苷和糖类。毫无疑问,这种方法为将碳硼烷部分快速掺入小分子提供了一种选择性途径,用于硼中子俘获治疗,这需要靶向输送富硼基团。
更新日期:2023-03-24
中文翻译:

B(9)-OH-o-Carboranes:合成、机理和性质探索
在此,我们通过在三氟甲磺酸 (HOTf) 和六氟异丙醇 (HFIP) 的协助下使用市售的 68% HNO 3氧化邻碳硼烷,提出了一种化学稳健且高效的 B(9)-OH-o-碳硼烷合成路线. 反应效率高,碳硼烷范围广,B(9)/B(8)的选择性高达98:2。这种转化的成功依赖于 HNO 3的强亲电性和氧化性,通过布朗斯台德酸 HOTf 和溶剂 HFIP 的氢键促进。机理研究表明,邻碳硼烷的氧化涉及 HNO 3的初始亲电攻击到邻碳硼烷的最具负电性的 B(9) 处的氢原子。在这种转化中,B-H键的氢原子是亲核位点,不同于亲电取代反应中硼原子是亲核位点。因此,这是邻碳硼烷在温和条件下的氧化还原反应,其中 N(V) → N(III) 和 H(-I) → H(I)。9-OH- o的衍生化-carborane 进一步检查,carboranyl 基团被成功地引入氨基酸、聚乙二醇、生物素、脱氧尿苷和糖类。毫无疑问,这种方法为将碳硼烷部分快速掺入小分子提供了一种选择性途径,用于硼中子俘获治疗,这需要靶向输送富硼基团。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号