Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-03-20 , DOI: 10.1016/j.bioorg.2023.106484
Yanhua Fan 1 , Feng Zhang 1 , Liang Xiong 1 , Mingzhi Su 1 , Fang Luo 1 , Mei Li 1 , Qing Li 1 , Ting Zhong 1 , Meitao Yuan 1 , Yongnan Xu 2 , Shuzhen Mu 1 , Huarong Yang 3
|
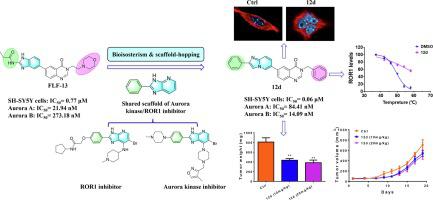
ROR1 and Aurora kinase were overexpressed in various cancers and essential for cell proliferation, survive and metastasis. Pharmaceutical inhibition of ROR1 and Aurora kinase abrogated the activation of downstream signaling and induced cancer cell apoptosis. Hence, ROR1 and Aurora kinase considered as attractive therapeutic targets for the development of anticancer drugs. In the present work, three series of novel 6-(imidazo[1,2-a] pyridin-6-yl)-quinazolin-4(3H)-one derivatives were designed and synthesized via bioisosterism and scaffold-hopping strategies guided by FLF-13, an Aurora kinase inhibitor we discovered earlier. Most of compounds in series 2 and series 3 showed submicromolar to nanomolar inhibitory activity against multiple cancer cell lines. More importantly, compounds 12d and 12f in series 3 showed nanomolar inhibitory activity against all test cancer cells. The most promising compound 12d exhibited potent inhibitory activity against Aurora A and Aurora B with IC50 values of 84.41 nM and 14.09 nM, respectively. Accordingly, compounds 12d induced G2/M phase cell cycle arrest at 24 h and polyploidy at 48 h. It's worth noting that 12d also displayed inhibitory activity against ROR1 and induce cell apoptosis. Furthermore, 12d could significantly inhibit the tumor growth in SH-SY5Y xenograft model with tumor growth inhibitory rate (IR) up to 46.31 % at 10 mg/kg and 52.66 % at 20 mg/kg. Overall, our data suggested that 12d might serve as a promising candidate for the development of therapeutic agents for cancers with aberrant expression of ROR1 and Aurora kinases by simultaneously targeting ROR1 and Aurora kinase.
中文翻译:

6-(imidazo[1,2-a] pyridin-6-yl) quinazolin-4(3H)-one 衍生物的设计、合成和生物学评价作为双重靶向 Aurora 激酶和 ROR1 的强效抗癌剂
ROR1 和 Aurora 激酶在多种癌症中过表达,对细胞增殖、存活和转移至关重要。ROR1 和 Aurora 激酶的药物抑制消除了下游信号的激活并诱导了癌细胞凋亡。因此,ROR1 和 Aurora 激酶被认为是开发抗癌药物的有吸引力的治疗靶点。在目前的工作中,三个系列的新型 6-(imidazo[1,2- a ] pyridin-6-yl)-quinazolin-4(3 H )-one 衍生物通过生物电子等排和脚手架跳跃策略被设计和合成FLF-13,我们之前发现的一种极光激酶抑制剂。系列 2 和系列 3 中的大多数化合物显示出对多种癌细胞系的亚微摩尔到纳摩尔抑制活性。更重要的是,化合物系列 3 中的12d和12f显示出对所有测试癌细胞的纳摩尔抑制活性。最有希望的化合物12d对 Aurora A 和 Aurora B 表现出有效的抑制活性,IC 50值分别为 84.41 nM 和 14.09 nM。因此,化合物12d在 24 小时诱导 G2/M 期细胞周期停滞,在 48 小时诱导多倍体。值得注意的是,12d还显示出对 ROR1 的抑制活性并诱导细胞凋亡。此外,12d可显着抑制 SH-SY5Y 异种移植模型中的肿瘤生长,肿瘤生长抑制率 (IR) 在 10 mg/kg 时高达 46.31%,在 20 mg/kg 时高达 52.66%。总体而言,我们的数据表明12d通过同时靶向 ROR1 和 Aurora 激酶,可能作为开发具有 ROR1 和 Aurora 激酶异常表达的癌症治疗剂的有前途的候选者。







































 京公网安备 11010802027423号
京公网安备 11010802027423号