当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flipping the Substrate Creates a Highly Selective Halohydrin Dehalogenase for the Synthesis of Chiral 4-Aryl-2-oxazolidinones from Readily Available Epoxides
ACS Catalysis ( IF 13.1 ) Pub Date : 2023-03-24 , DOI: 10.1021/acscatal.2c06417
Chuanhua Zhou , Xi Chen , Tong Lv , Xu Han , Jinhui Feng , Weidong Liu , Qiaqing Wu , Dunming Zhu
ACS Catalysis ( IF 13.1 ) Pub Date : 2023-03-24 , DOI: 10.1021/acscatal.2c06417
Chuanhua Zhou , Xi Chen , Tong Lv , Xu Han , Jinhui Feng , Weidong Liu , Qiaqing Wu , Dunming Zhu
|
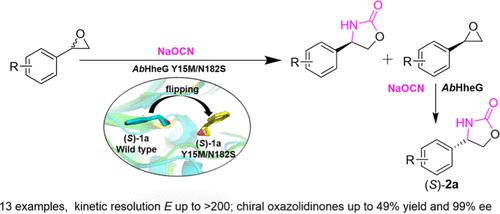
Chiral oxazolidinones are a class of important heterocyclic compounds in pharmaceutical chemistry due to their biological activity. Halohydrin dehalogenase-catalyzed epoxide ring-opening reaction with cyanate offers an attractive approach to the synthesis of chiral oxazolidinones, but the α/β-regioselectivity and stereoselectivity are still un-addressed issues. In this study, a unique halohydrin dehalogenase (AbHheG) was found to have high activity and α/β-regioselectivity toward the ring opening of racemic styrene oxide with cyanate but with poor stereoselectivity (E < 3). By reshaping the substrate-binding site of AbHheG, a variant Y15M/N182S was obtained with excellent α/β-regioselectivity and stereoselectivity. The variant showed E > 200 for 9 of the 13 tested styrene oxides. Since (R)-4-aryl-2-oxazolidinones were easily separated from (R)-styrene oxides, both (R)- and (S)-4-aryl-2-oxazolidinones could be readily prepared. Crystallographic and enzyme–substrate docking analysis showed that flipping of the substrate in the binding site resulted in the R-configuration substrate being away from the catalytic triad in the mutant, which was responsible for the enhanced α/β-regioselectivity and stereoselectivity. This work has demonstrated that halohydrin dehalogenase is a useful biocatalyst for the synthesis of both enantiomers of 4-aryl-2-oxazolidinones from readily available racemic styrene oxides. The structural and computational studies provide a guidance for further engineering of halohydrin dehalogenases to control the α/β-regioselectivity and stereoselectivity for the efficient synthesis of the desired optically pure 4- or 5-substituted 2-oxazolidinones.
中文翻译:

翻转底物产生高选择性卤代醇脱卤酶,用于从现成的环氧化物中合成手性 4-Aryl-2-oxazolidinones
手性恶唑烷酮类化合物因其生物活性而成为药物化学中一类重要的杂环化合物。卤代醇脱卤酶催化的环氧化物与氰酸酯的开环反应为合成手性恶唑烷酮提供了一种有吸引力的方法,但 α/β-区域选择性和立体选择性仍然是未解决的问题。在这项研究中,发现一种独特的卤代醇脱卤素酶 ( Ab HheG) 对外消旋氧化苯乙烯与氰酸盐的开环具有高活性和 α/β-区域选择性,但立体选择性较差 ( E < 3)。通过重塑Ab HheG的底物结合位点,获得了具有优异 α/β-区域选择性和立体选择性的变体 Y15M/N182S。变体显示E> 200 对于 13 种测试的氧化苯乙烯中的 9 种。由于 ( R )-4-aryl-2-oxazolidinones 很容易与 ( R )-styrene oxides 分离,因此 ( R )- 和 ( S )-4-aryl-2-oxazolidinones 都可以很容易地制备。晶体学和酶-底物对接分析表明,翻转结合位点的底物会导致R-构型底物远离突变体中的催化三联体,这是增强α/β-区域选择性和立体选择性的原因。这项工作表明,卤代醇脱卤酶是一种有用的生物催化剂,可用于从容易获得的外消旋苯乙烯氧化物合成 4-aryl-2-oxazolidinones 的两种对映体。结构和计算研究为卤代醇脱卤酶的进一步工程化提供指导,以控制 α/β-区域选择性和立体选择性,从而有效合成所需的光学纯 4-或 5-取代的 2-恶唑烷酮。
更新日期:2023-03-24
中文翻译:

翻转底物产生高选择性卤代醇脱卤酶,用于从现成的环氧化物中合成手性 4-Aryl-2-oxazolidinones
手性恶唑烷酮类化合物因其生物活性而成为药物化学中一类重要的杂环化合物。卤代醇脱卤酶催化的环氧化物与氰酸酯的开环反应为合成手性恶唑烷酮提供了一种有吸引力的方法,但 α/β-区域选择性和立体选择性仍然是未解决的问题。在这项研究中,发现一种独特的卤代醇脱卤素酶 ( Ab HheG) 对外消旋氧化苯乙烯与氰酸盐的开环具有高活性和 α/β-区域选择性,但立体选择性较差 ( E < 3)。通过重塑Ab HheG的底物结合位点,获得了具有优异 α/β-区域选择性和立体选择性的变体 Y15M/N182S。变体显示E> 200 对于 13 种测试的氧化苯乙烯中的 9 种。由于 ( R )-4-aryl-2-oxazolidinones 很容易与 ( R )-styrene oxides 分离,因此 ( R )- 和 ( S )-4-aryl-2-oxazolidinones 都可以很容易地制备。晶体学和酶-底物对接分析表明,翻转结合位点的底物会导致R-构型底物远离突变体中的催化三联体,这是增强α/β-区域选择性和立体选择性的原因。这项工作表明,卤代醇脱卤酶是一种有用的生物催化剂,可用于从容易获得的外消旋苯乙烯氧化物合成 4-aryl-2-oxazolidinones 的两种对映体。结构和计算研究为卤代醇脱卤酶的进一步工程化提供指导,以控制 α/β-区域选择性和立体选择性,从而有效合成所需的光学纯 4-或 5-取代的 2-恶唑烷酮。






















































 京公网安备 11010802027423号
京公网安备 11010802027423号