当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Process Development for a 1H-Indazole Synthesis Using an Intramolecular Ullmann-Type Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.joc.2c02771 Jon I Day 1 , Katherine N Allen-Moyer 2 , Kevin P Cole 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.joc.2c02771 Jon I Day 1 , Katherine N Allen-Moyer 2 , Kevin P Cole 1
Affiliation
|
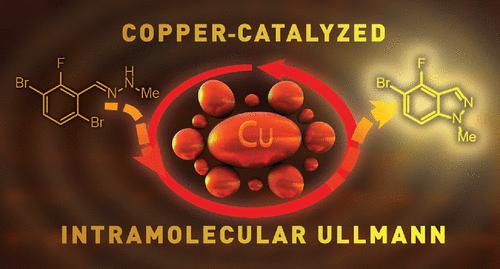
Within the scope of developing a new route to an active pharmaceutical ingredient intermediate, we had need of a fluorinated indazole. Although an established route was in place, it was undesirable due to safety and selectivity concerns. A concise and improved route was developed to form the desired indazole, which takes advantage of an electronically directed metalation/formylation sequence followed by condensation with methyl hydrazine to form a hydrazone and culminates in a copper-catalyzed intramolecular Ullmann cyclization. The Ullmann reaction was plagued with difficulties ranging from poor reactivity to thermal hazard concerns, but use of high-throughput screening, statistical modeling, and an unusual isolation method for fine chemicals, safe and optimal conditions were found that produce high-purity isolated material in excellent yields at a laboratory scale.
中文翻译:

使用分子内 Ullmann 型反应的 1H-吲唑合成工艺开发
在开发活性药物成分中间体新途径的范围内,我们需要氟化吲唑。尽管已经制定了一条既定路线,但出于安全性和选择性方面的考虑,这是不受欢迎的。开发了一种简明和改进的路线来形成所需的吲唑,它利用电子引导的金属化/甲酰化序列,然后与甲基肼缩合形成腙,并最终导致铜催化的分子内乌尔曼环化。乌尔曼反应遇到了从反应性差到热危害问题等各种困难,但使用高通量筛选、统计建模和一种不寻常的精细化学品分离方法,
更新日期:2023-03-23
中文翻译:

使用分子内 Ullmann 型反应的 1H-吲唑合成工艺开发
在开发活性药物成分中间体新途径的范围内,我们需要氟化吲唑。尽管已经制定了一条既定路线,但出于安全性和选择性方面的考虑,这是不受欢迎的。开发了一种简明和改进的路线来形成所需的吲唑,它利用电子引导的金属化/甲酰化序列,然后与甲基肼缩合形成腙,并最终导致铜催化的分子内乌尔曼环化。乌尔曼反应遇到了从反应性差到热危害问题等各种困难,但使用高通量筛选、统计建模和一种不寻常的精细化学品分离方法,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号