Cancer Cell ( IF 48.8 ) Pub Date : 2023-03-23 , DOI: 10.1016/j.ccell.2023.03.002
Wei Zhou 1 , Heng Liu 1 , Zhe Yuan 1 , Joseph Zundell 1 , Martina Towers 2 , Jianhuang Lin 1 , Simona Lombardi 3 , Hao Nie 1 , Brennah Murphy 1 , Tyler Yang 1 , Chen Wang 1 , Liping Liao 1 , Aaron R Goldman 4 , Toshitha Kannan 5 , Andrew V Kossenkov 6 , Ronny Drapkin 7 , Luis J Montaner 1 , Daniel T Claiborne 1 , Nan Zhang 1 , Shuai Wu 1 , Rugang Zhang 8
|
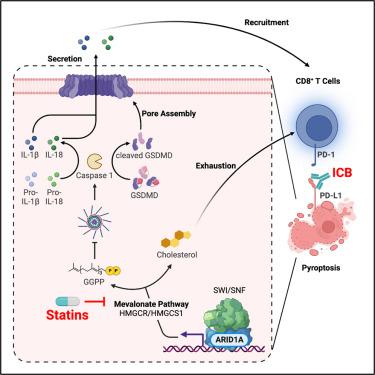
ARID1A, encoding a subunit of the SWI/SNF complex, is mutated in ∼50% of clear cell ovarian carcinoma (OCCC) cases. Here we show that inhibition of the mevalonate pathway synergizes with immune checkpoint blockade (ICB) by driving inflammasome-regulated immunomodulating pyroptosis in ARID1A-inactivated OCCCs. SWI/SNF inactivation downregulates the rate-limiting enzymes in the mevalonate pathway such as HMGCR and HMGCS1, which creates a dependence on the residual activity of the pathway in ARID1A-inactivated cells. Inhibitors of the mevalonate pathway such as simvastatin suppresses the growth of ARID1A mutant, but not wild-type, OCCCs. In addition, simvastatin synergizes with anti-PD-L1 antibody in a genetic OCCC mouse model driven by conditional Arid1a inactivation and in a humanized immunocompetent ARID1A mutant patient-derived OCCC mouse model. Our data indicate that inhibition of the mevalonate pathway simultaneously suppresses tumor cell growth and boosts antitumor immunity by promoting pyroptosis, which synergizes with ICB in suppressing ARID1A-mutated cancers.
中文翻译:

靶向甲羟戊酸途径通过促进焦亡来抑制 ARID1A 失活的癌症
ARID1A编码 SWI/SNF 复合体的一个亚基,在约 50% 的透明细胞卵巢癌 (OCCC) 病例中发生突变。在这里,我们表明,甲羟戊酸途径的抑制与免疫检查点阻断(ICB)协同作用,通过驱动ARID1A失活的 OCCC 中炎症小体调节的免疫调节焦亡。 SWI/SNF 失活下调甲羟戊酸途径中的限速酶,例如 HMGCR 和 HMGCS1,这在ARID1A失活细胞中产生对该途径残留活性的依赖性。甲羟戊酸途径抑制剂(例如辛伐他汀)可抑制ARID1A突变体的生长,但不会抑制野生型 OCCC 的生长。此外,在由条件性Arid1a失活驱动的遗传 OCCC 小鼠模型和人源化免疫活性ARID1A突变患者衍生的 OCCC 小鼠模型中,辛伐他汀与抗 PD-L1 抗体具有协同作用。我们的数据表明,抑制甲羟戊酸途径可同时抑制肿瘤细胞生长,并通过促进焦亡来增强抗肿瘤免疫力,这与 ICB 协同抑制ARID1A突变的癌症。

































 京公网安备 11010802027423号
京公网安备 11010802027423号