当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Equilibrium at the HCOOH-Saturated TiO2(110)–Water Interface
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.jpclett.2c03788 Fernanda Brandalise Nunes 1 , Nicolò Comini 2, 3 , J Trey Diulus 2, 3 , Thomas Huthwelker 3 , Marcella Iannuzzi 1 , Jürg Osterwalder 2 , Zbynek Novotny 2, 3, 4
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-03-23 , DOI: 10.1021/acs.jpclett.2c03788 Fernanda Brandalise Nunes 1 , Nicolò Comini 2, 3 , J Trey Diulus 2, 3 , Thomas Huthwelker 3 , Marcella Iannuzzi 1 , Jürg Osterwalder 2 , Zbynek Novotny 2, 3, 4
Affiliation
|
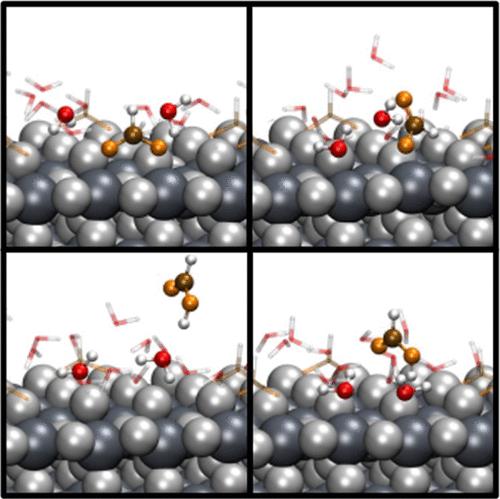
Carboxylic acids bind to titanium dioxide (TiO2) dissociatively, forming surface superstructures that give rise to a (2 × 1) pattern detected by low-energy electron diffraction. Exposing this system to water, however, leads to a loss of the highly ordered surface structure. The formate-covered surface was investigated by a combination of diffraction and spectroscopy techniques, together with static and dynamic ab initio simulations, with the conclusion that a dynamic equilibrium exists between adsorbed formic acid and water molecules. This equilibrium process is an important factor for obtaining a better understanding of controlling the self-cleaning properties of TiO2, because the formic acid monolayer is responsible for the amphiphilic character of the surface.
中文翻译:

HCOOH-饱和 TiO2(110)-水界面的动态平衡
羧酸与二氧化钛 (TiO 2 ) 解离结合,形成表面超结构,产生 (2 × 1) 图案,可通过低能电子衍射检测到。然而,将该系统暴露在水中会导致高度有序的表面结构丢失。通过结合衍射和光谱技术以及静态和动态从头算模拟研究甲酸盐覆盖的表面,得出的结论是吸附的甲酸和水分子之间存在动态平衡。该平衡过程是更好地理解控制 TiO 2自清洁特性的重要因素,因为甲酸单层负责表面的两亲特性。
更新日期:2023-03-23
中文翻译:

HCOOH-饱和 TiO2(110)-水界面的动态平衡
羧酸与二氧化钛 (TiO 2 ) 解离结合,形成表面超结构,产生 (2 × 1) 图案,可通过低能电子衍射检测到。然而,将该系统暴露在水中会导致高度有序的表面结构丢失。通过结合衍射和光谱技术以及静态和动态从头算模拟研究甲酸盐覆盖的表面,得出的结论是吸附的甲酸和水分子之间存在动态平衡。该平衡过程是更好地理解控制 TiO 2自清洁特性的重要因素,因为甲酸单层负责表面的两亲特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号