Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.jcis.2023.03.101 Alfonso Cabezón 1 , Martin Calvelo 2 , Juan R Granja 1 , Ángel Piñeiro 3 , Rebeca Garcia-Fandino 1
|
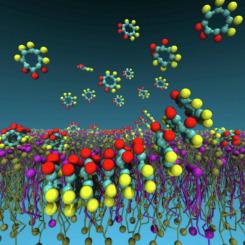
Cyclic peptides (CPs) formed by alternation of D- and L-amino acids (D,L-CPs) can self-assemble into nanotubes (SCPNs) by parallel or/and antiparallel stacking. Different applications have been attributed to these nanotubes, including the disruption of lipid bilayers of specific compositions and the selective transport of ions throughout membranes. Molecular dynamics (MD) simulations have significantly contributed to understand the interaction between CPs, including the structural, dynamic and transport properties of their supramolecular aggregates. The high computational cost of atomic resolution forcefields makes them impractical for simulating the self-assembly of macromolecules, so coarse-grained (CG) models might represent a more feasible solution for this purpose. However, general CG models used for the simulation of biomolecules such as the MARTINI forcefield do not explicitly consider the non-covalent interactions leading to the formation of secondary structure patterns in proteins. This becomes particularly important in the case of CPs due to the D- and L-chirality alternation in their sequence, leading to opposite orientations of the backbone polar groups on both sides of the cyclic ring plane. In order to overcome this limitation, we have extended the MARTINI forcefield to introduce chirality in each residue of the CPs. The new parametrization, which we have called MA(R/S)TINI, reproduces the expected self-assembly patterns for several CP sequences in the presence of different membrane models, explicitly considering the chirality of the CPs and with no significant extra computational cost. Our simulations provide new mechanistic information of how these systems self-assemble in presence of different lipid scenarios, showing that the CP-CP and CP-membrane interactions are sensitive to the peptide sequence chirality. This opens the door to design new bioactive CPs based on CG-MD simulations. A web-based tool for the automatic parameterization of new CP sequences using MA(R/S)TINI, among other functionalities, is under construction (see http://cyclopep.com).
中文翻译:

利用手性感知 MA(R/S)TINI 力场揭示膜中环肽自组装的机制
由D - 和L -氨基酸交替形成的环肽 (CP) ( D,L-CPs) 可以通过平行或/和反平行堆叠自组装成纳米管 (SCPNs)。这些纳米管有不同的应用,包括破坏特定成分的脂质双层和离子在整个膜中的选择性传输。分子动力学 (MD) 模拟为理解 CP 之间的相互作用做出了重大贡献,包括其超分子聚集体的结构、动力学和传输特性。原子分辨率力场的高计算成本使其无法模拟大分子的自组装,因此粗粒度 (CG) 模型可能代表了用于此目的的更可行的解决方案。然而,用于模拟生物分子(如 MARTINI 力场)的一般 CG 模型没有明确考虑导致蛋白质二级结构模式形成的非共价相互作用。这在 CP 的情况下变得尤为重要,因为D - 和L-它们序列中的手性交替,导致环平面两侧的骨架极性基团的相反方向。为了克服这个限制,我们扩展了 MARTINI 力场以在 CP 的每个残基中引入手性。我们称之为 MA(R/S)TINI 的新参数化在存在不同膜模型的情况下再现了多个 CP 序列的预期自组装模式,明确考虑了 CP 的手性并且没有显着的额外计算成本。我们的模拟提供了这些系统如何在存在不同脂质情况下自组装的新机制信息,表明 CP-CP 和 CP-膜相互作用对肽序列手性敏感。这为基于 CG-MD 模拟设计新的生物活性 CP 打开了大门。http://cyclopep.com)。










































 京公网安备 11010802027423号
京公网安备 11010802027423号