European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.ejmech.2023.115296 Irina V Sandulenko 1 , Irina V Belozertseva 2 , Edwin E Zvartau 2 , Maria V Zelentsova 1 , Asmik A Ambartsumyan 1 , Alexander F Smol'yakov 3 , Sergey K Moiseev 1
|
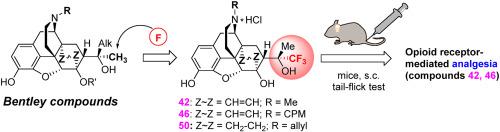
Thevinols and their 3-O-demethylated relatives, orvinols, are derivatives of the Diels-Alder adduct of natural alkaloid thebaine with methyl vinyl ketone. Taken together, thevinols and orvinols constitute an important family of opioid receptor (OR) ligands playing an important role in both the OR mediated antinociception and OR antagonism. Herein, we disclose for the first time the OR activity of orvinols fluorinated within the pharmocophore associated with C(20) and its surrounding along with a dependence of the activity profile on the substituent at N(17). Starting from thevinone and 18,19-dihydrothevinone, a family of C(21)-fluorinated orvinols bearing methyl, cyclopropylmethyl (CPM), and allyl substituent at N(17) was synthesized. The fluorinated compounds were evaluated for OR activity. The orvinols bearing three fluorine atoms at C(21) were found to retain the properties of OR ligands and their activity profile depends on the substituent at N(17). Pilot in vivo experiments in a model of acute pain (tail-flick test in mice) revealed that 6-O-desmethyl-21,21,21-trifluoro-20-methylorvinol at doses 1.0–10.0 mg/kg (s.c.) exhibits analgesic activity at the level of morphine for a duration of 30–180 min. Its N(17)-CPM counterpart demonstrated the partial opioid agonist properties. The N(17)-allyl substituted derivative showed no analgesic activity. In vivo evaluation of an analgesic activity indicates that 21,21,21-trifluoro-20-methylorvinols represent a novel family of OR ligands related to buprenorphine, diprenorphine, etc. These compounds are promising for the structure-activity relationship studies among the thevinol/orvinol series as well as for a search for new OR ligands with potentially valuable pharmacological profiles.
中文翻译:

用于阿片类药物配体的 C(21)-氟化 thevinol 支架。21,21,21-Trifluoro-6-O-nororvinols:设计、合成和镇痛活性
Thevinols 及其 3- O-去甲基化的亲属,orvinols,是天然生物碱蒂巴因与甲基乙烯基酮的 Diels-Alder 加合物的衍生物。总之,thevinols 和 orvinols 构成一个重要的阿片受体 (OR) 配体家族,在 OR 介导的抗伤害作用和 OR 拮抗作用中发挥重要作用。在此,我们首次公开了与 C(20) 及其周围相关的药效团内氟化的 orvinols 的 OR 活性,以及活性谱对 N(17) 取代基的依赖性。从 thevinone 和 18,19-dihydrothevinone 开始,在 N(17) 上合成了一个带有甲基、环丙基甲基 (CPM) 和烯丙基取代基的 C(21)-氟化 orvinols 家族。评估氟化化合物的 OR 活性。发现在 C(21) 处带有三个氟原子的 orvinols 保留了 OR 配体的特性,并且它们的活性分布取决于 N(17) 处的取代基。飞行员急性疼痛模型的体内实验(小鼠甩尾试验)显示 6- O -desmethyl-21,21,21-trifluoro-20-methylorvinol 在剂量为 1.0–10.0 mg/kg (sc) 时表现出镇痛活性在吗啡水平持续 30-180 分钟。它的N (17)-CPM 对应物显示出部分阿片类激动剂特性。N ( 17)-烯丙基取代的衍生物没有镇痛活性。体内对镇痛活性的评估表明,21,21,21-三氟-20-甲基奥维诺代表了一个与丁丙诺啡、二丙诺啡等相关的新型 OR 配体家族。这些化合物有望用于 thevinol/orvinol 系列之间的构效关系研究以及寻找具有潜在药理学价值的新 OR 配体。































 京公网安备 11010802027423号
京公网安备 11010802027423号