当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cd(II) complexes derived from thiazoline, hydrazide and carbodithioate ligands: synthesis, crystal structures and electrochemical sensing of uric acid
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2023-03-22 , DOI: 10.1002/aoc.7085
Shubham Jaiswal 1 , Shivendra Kumar Pandey 1 , Jyoti Prajapati 1 , S. Chandra 1 , M. K. Gond 1 , M. K. Bharty 1 , Ida Tiwari 1 , R. J. Butcher 2
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2023-03-22 , DOI: 10.1002/aoc.7085
Shubham Jaiswal 1 , Shivendra Kumar Pandey 1 , Jyoti Prajapati 1 , S. Chandra 1 , M. K. Gond 1 , M. K. Bharty 1 , Ida Tiwari 1 , R. J. Butcher 2
Affiliation
|
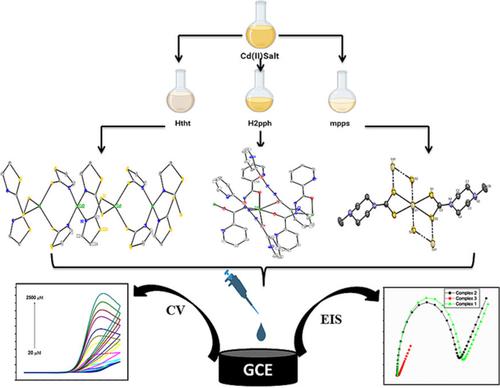
Three novel Cd(II) metal complexes 1–3 have been synthesized with different derivatives of thiazoline, hydrazide and carbodithioate. These metal complexes are abbreviated as [Cd (tht)2]n, [Cd (Hpph)2]n and [Cd (mpps)2]n along with their respective ligands Htht, H2pph and mpps. The developed metal complexes are characterized using X-ray diffraction (XRD) data and other spectroscopic techniques (Infrared [IR], nuclear magnetic resonance [NMR], ultraviolet–visible [UV–vis] and fluorescence). The polymeric nature of these cadmium complexes have been ascertained by single XRD data. Several significant interactions were also revealed, which aid in the stabilization of these complexes' supramolecular architecture. Comparing complex 2 with complexes 1 and 3, the absorption spectra of complex 2 exhibits a greater λmax. On comparing the fluorescence study of these complexes, complex 2 has a higher fluorescence than complexes 1 and 3. The cyclic voltammetry (CV) approach was employed to detect the electrochemical behaviour of these complexes, as well as the sensing of uric acid (UA) by these complexes via modified glassy carbon electrodes (GCEs). According to the conclusions received by CV study, the modified electrode containing complex 3 has admirable UA electro catalytic activity. This UA electrochemical sensing device offers a low detection limit (0.3 μM), fine linear ranges (30–1500 μM), reasonable sensitivity, and a fast reaction time.
中文翻译:

来自噻唑啉、酰肼和二硫代碳酸盐配体的 Cd(II) 配合物:尿酸的合成、晶体结构和电化学传感
三种新型 Cd(II) 金属配合物1-3已由噻唑啉、酰肼和二硫代碳酸盐的不同衍生物合成。这些金属络合物缩写为 [Cd (tht) 2 ] n、[Cd (Hpph) 2 ] n和 [Cd (mpps) 2 ] n以及它们各自的配体 Htht、H 2pph 和 mpps。使用 X 射线衍射 (XRD) 数据和其他光谱技术(红外 [IR]、核磁共振 [NMR]、紫外-可见 [UV-vis] 和荧光)对开发的金属配合物进行表征。这些镉络合物的聚合物性质已通过单个 XRD 数据确定。还揭示了几种重要的相互作用,这有助于稳定这些复合物的超分子结构。将络合物2与络合物1和3进行比较,络合物2的吸收光谱表现出更大的λmax。比较这些配合物的荧光研究,配合物2比配合物1和3具有更高的荧光. 采用循环伏安法 (CV) 方法检测这些复合物的电化学行为,以及这些复合物通过改性玻碳电极 (GCE) 检测尿酸 (UA)。根据CV研究得到的结论,含有络合物3的修饰电极具有良好的UA电催化活性。这种 UA 电化学传感装置具有低检测限 (0.3 μM)、良好的线性范围 (30–1500 μM)、合理的灵敏度和快速的反应时间。
更新日期:2023-03-22
中文翻译:

来自噻唑啉、酰肼和二硫代碳酸盐配体的 Cd(II) 配合物:尿酸的合成、晶体结构和电化学传感
三种新型 Cd(II) 金属配合物1-3已由噻唑啉、酰肼和二硫代碳酸盐的不同衍生物合成。这些金属络合物缩写为 [Cd (tht) 2 ] n、[Cd (Hpph) 2 ] n和 [Cd (mpps) 2 ] n以及它们各自的配体 Htht、H 2pph 和 mpps。使用 X 射线衍射 (XRD) 数据和其他光谱技术(红外 [IR]、核磁共振 [NMR]、紫外-可见 [UV-vis] 和荧光)对开发的金属配合物进行表征。这些镉络合物的聚合物性质已通过单个 XRD 数据确定。还揭示了几种重要的相互作用,这有助于稳定这些复合物的超分子结构。将络合物2与络合物1和3进行比较,络合物2的吸收光谱表现出更大的λmax。比较这些配合物的荧光研究,配合物2比配合物1和3具有更高的荧光. 采用循环伏安法 (CV) 方法检测这些复合物的电化学行为,以及这些复合物通过改性玻碳电极 (GCE) 检测尿酸 (UA)。根据CV研究得到的结论,含有络合物3的修饰电极具有良好的UA电催化活性。这种 UA 电化学传感装置具有低检测限 (0.3 μM)、良好的线性范围 (30–1500 μM)、合理的灵敏度和快速的反应时间。

































 京公网安备 11010802027423号
京公网安备 11010802027423号