当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonthermal Plasma-Assisted CO2-H2O Conversion over NiO and Co3O4 Supported on CeO2
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2023-03-21 , DOI: 10.1002/ceat.202200481
Abhinav Bajpai 1 , Sushant Kumar 1
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2023-03-21 , DOI: 10.1002/ceat.202200481
Abhinav Bajpai 1 , Sushant Kumar 1
Affiliation
|
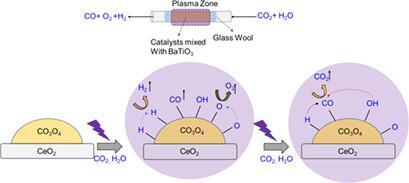
Transformation of CO2 into value-added chemicals remains a key challenge. Herein, nonthermal plasma (NTP)-activated CO2-H2O conversion over BaTiO3-mixed NiO/CeO2 (Ni/Ce+) and Co3O4/CeO2 (Co/Ce+) catalysts is demonstrated. In a continuous-flow dielectric barrier discharge reactor and under ambient conditions, optimum Co/Ce+ showed formation rates of CO and O2 of (43.6 ± 3.1) and (20.8 ± 0.7) μmol g−1h−1, respectively. CO2 conversion was found to be dependent on a combination of the basicity of the catalyst surface, CO2 flow rate, plasma input power, and relative humidity. Subsequently, kinetic analysis suggested that the activation energies over Ni/Ce+, Co/Ce+, and NiCo/Ce+ are 35.68, 15.61, and 25.88 kJ mol−1, respectively. By combining these findings, a plausible reaction mechanism that can facilitate understanding the trend of product formation is also provided.
中文翻译:

CeO2 负载 NiO 和 Co3O4 的非热等离子体辅助 CO2-H2O 转化
将CO 2转化为增值化学品仍然是一个关键挑战。在此,证明了BaTiO 3混合的NiO/CeO 2 (Ni/Ce + )和Co 3 O 4 /CeO 2 (Co/Ce + )催化剂上的非热等离子体(NTP)激活的CO 2 -H 2 O转化。在连续流介质阻挡放电反应器和环境条件下,最佳Co/Ce +显示CO和O 2的形成速率分别为(43.6±3.1)和(20.8±0.7)μmol g -1 h -1。二氧化碳_发现转化率取决于催化剂表面的碱度、CO 2流速、等离子体输入功率和相对湿度的组合。随后,动力学分析表明Ni/Ce +、Co/Ce +和NiCo/Ce +的活化能分别为35.68、15.61和25.88 kJ mol -1。通过结合这些发现,还提供了一种可能的反应机制,有助于理解产物形成的趋势。
更新日期:2023-03-21
中文翻译:

CeO2 负载 NiO 和 Co3O4 的非热等离子体辅助 CO2-H2O 转化
将CO 2转化为增值化学品仍然是一个关键挑战。在此,证明了BaTiO 3混合的NiO/CeO 2 (Ni/Ce + )和Co 3 O 4 /CeO 2 (Co/Ce + )催化剂上的非热等离子体(NTP)激活的CO 2 -H 2 O转化。在连续流介质阻挡放电反应器和环境条件下,最佳Co/Ce +显示CO和O 2的形成速率分别为(43.6±3.1)和(20.8±0.7)μmol g -1 h -1。二氧化碳_发现转化率取决于催化剂表面的碱度、CO 2流速、等离子体输入功率和相对湿度的组合。随后,动力学分析表明Ni/Ce +、Co/Ce +和NiCo/Ce +的活化能分别为35.68、15.61和25.88 kJ mol -1。通过结合这些发现,还提供了一种可能的反应机制,有助于理解产物形成的趋势。


































 京公网安备 11010802027423号
京公网安备 11010802027423号