当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Silylation of Electron-Deficient Heterocycles Using N-Hydroxyphthalimide as HAT Catalyst
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-03-21 , DOI: 10.1002/adsc.202300112
Cong Jiang 1 , Yujie Liao 1 , Heng Li 1 , Sumin Zhang 1 , Ping Liu 1 , Peipei Sun 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-03-21 , DOI: 10.1002/adsc.202300112
Cong Jiang 1 , Yujie Liao 1 , Heng Li 1 , Sumin Zhang 1 , Ping Liu 1 , Peipei Sun 1
Affiliation
|
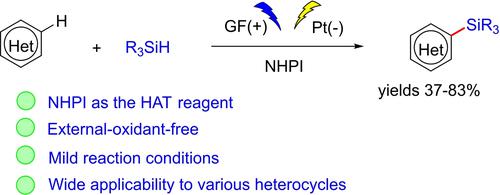
A practical electrochemical Minisci-type reaction for the synthesis of silylated heterocycles is described. Using N-hydroxyphthalimide (NHPI) as the hydrogen atom transfer (HAT) catalyst and trialkylsilanes as the silyl radical sources, a wide range of electron-deficient heterocycles in-cluding quinoline, quinoxaline, phthalazine, 3,6-dichloropyridazine, phenanthridine, 6-chloroimidazo[1,2-b]pyridazine and coumarin, etc. were easily converted to the corresponding silylated products in 37–83% yields.
中文翻译:

N-羟基邻苯二甲酰亚胺作为 HAT 催化剂对缺电子杂环进行电化学硅烷化反应
描述了用于合成甲硅烷基化杂环的实用电化学 Minisci 型反应。使用N-羟基邻苯二甲酰亚胺 (NHPI) 作为氢原子转移 (HAT) 催化剂和三烷基硅烷作为甲硅烷基自由基来源,广泛的缺电子杂环包括喹啉、喹喔啉、酞嗪、3,6-二氯哒嗪、菲啶、6 -chloroimidazo[1,2- b ]哒嗪和香豆素等很容易转化为相应的甲硅烷基化产物,收率为37-83%。
更新日期:2023-03-21
中文翻译:

N-羟基邻苯二甲酰亚胺作为 HAT 催化剂对缺电子杂环进行电化学硅烷化反应
描述了用于合成甲硅烷基化杂环的实用电化学 Minisci 型反应。使用N-羟基邻苯二甲酰亚胺 (NHPI) 作为氢原子转移 (HAT) 催化剂和三烷基硅烷作为甲硅烷基自由基来源,广泛的缺电子杂环包括喹啉、喹喔啉、酞嗪、3,6-二氯哒嗪、菲啶、6 -chloroimidazo[1,2- b ]哒嗪和香豆素等很容易转化为相应的甲硅烷基化产物,收率为37-83%。































 京公网安备 11010802027423号
京公网安备 11010802027423号