当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Diphenylamine on Binary Systems of Acetone + Ethanol, Acetone + Cyclohexane, and Ethanol + Cyclohexane at 101.3 kPa: Vapor–Liquid Equilibrium Measurement and Molecular Simulation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-03-22 , DOI: 10.1021/acs.jced.2c00663 Zhiping Du 1, 2 , Yu Fang 1 , Xiaogang Bi 1 , Sicheng Liu 1 , Yigang Ding 1, 2 , Zhiguo Yan 1, 2 , Xiaojun Yang 1, 2 , Xia Yin 1, 2 , Jie Liu 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-03-22 , DOI: 10.1021/acs.jced.2c00663 Zhiping Du 1, 2 , Yu Fang 1 , Xiaogang Bi 1 , Sicheng Liu 1 , Yigang Ding 1, 2 , Zhiguo Yan 1, 2 , Xiaojun Yang 1, 2 , Xia Yin 1, 2 , Jie Liu 1, 2
Affiliation
|
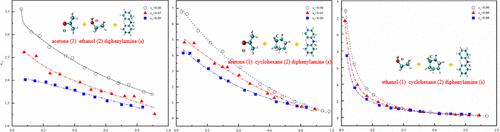
The recovery of organic solvents is important in the process of extracting nitrocellulose from waste propellants. Until now, the influence of diphenylamine from waste propellants on organic solvents has not been clear, which is a critical factor for their separation. In this paper, binary and ternary vapor–liquid equilibrium systems referring to mixtures containing diphenylamine, acetone, ethanol, and cyclohexane at 101.3 kPa were investigated systemically. The experimental results shows that diphenylamine increased the equilibrium temperature, decreased the relative volatility of organic solvents, and has a limited effect on the azeotropic composition for the systems of acetone + cyclohexane and ethanol + cyclohexane. In addition, a molecular dynamics analysis through GROMACS software was adopted to explore the interaction between diphenylamine and the above solvents, and the results show that the order of magnitude of the interaction force is: acetone is greater than ethanol and ethanol is greater than cyclohexane. This might be the main reason for the decreasing relative volatility of organic solvents.
中文翻译:

101.3 kPa 下二苯胺对丙酮 + 乙醇、丙酮 + 环己烷和乙醇 + 环己烷二元体系的影响:气液平衡测量和分子模拟
有机溶剂的回收在从废推进剂中提取硝化纤维的过程中具有重要意义。迄今为止,废推进剂中的二苯胺对有机溶剂的影响尚不清楚,这是它们分离的关键因素。在本文中,系统地研究了二元和三元汽液平衡系统,这些系统指的是在 101.3 kPa 下含有二苯胺、丙酮、乙醇和环己烷的混合物。实验结果表明,二苯胺提高了平衡温度,降低了有机溶剂的相对挥发度,对丙酮+环己烷和乙醇+环己烷体系的共沸组成影响有限。此外,采用GROMACS软件进行分子动力学分析探讨二苯胺与上述溶剂的相互作用,结果表明相互作用力的数量级为:丙酮大于乙醇,乙醇大于环己烷。这可能是有机溶剂相对挥发性降低的主要原因。
更新日期:2023-03-22
中文翻译:

101.3 kPa 下二苯胺对丙酮 + 乙醇、丙酮 + 环己烷和乙醇 + 环己烷二元体系的影响:气液平衡测量和分子模拟
有机溶剂的回收在从废推进剂中提取硝化纤维的过程中具有重要意义。迄今为止,废推进剂中的二苯胺对有机溶剂的影响尚不清楚,这是它们分离的关键因素。在本文中,系统地研究了二元和三元汽液平衡系统,这些系统指的是在 101.3 kPa 下含有二苯胺、丙酮、乙醇和环己烷的混合物。实验结果表明,二苯胺提高了平衡温度,降低了有机溶剂的相对挥发度,对丙酮+环己烷和乙醇+环己烷体系的共沸组成影响有限。此外,采用GROMACS软件进行分子动力学分析探讨二苯胺与上述溶剂的相互作用,结果表明相互作用力的数量级为:丙酮大于乙醇,乙醇大于环己烷。这可能是有机溶剂相对挥发性降低的主要原因。






























 京公网安备 11010802027423号
京公网安备 11010802027423号