Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
临界胶束浓度的精确定义及其在膜蛋白研究中使用的烷基麦芽糖苷的应用
RSC Advances ( IF 3.9 ) Pub Date : 2023-03-22 , DOI: 10.1039/d2ra07440k
Adrian Bothe 1 , Athina Zouni 1 , Frank Müh 2
Affiliation
|
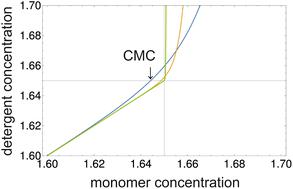
非离子洗涤剂的临界胶束浓度 (CMC) 定义为单体浓度与总洗涤剂浓度的函数关系的临界点,通过将该函数的三阶导数设置为零来确定。结合胶束形成的质量作用模型,该定义给出了 CMC 处单体与总洗涤剂浓度比的解析公式以及 CMC 与胶束化自由能g mic之间的关系。如果使用 8-苯胺基-1-萘磺酸 (ANS) 或类似探针染料的荧光增强来监测胶束形成,则理论断裂点与实验滴定曲线的断裂点一致。应用到一系列烷基链碳原子数为 8 至 12 的正烷基-β- D-麦芽糖苷,证明了分子热力学模型的良好性能,其中胶束化的自由能由g给出mic = σΦ + g pack + g st 。在该模型中, σ是表面张力量纲的拟合参数, Φ表示与水相接触的疏水分子表面面积的变化, g pack和g st分别是烷基链堆积的贡献。胶束内部和洗涤剂头部基团的空间排斥。 对不同来源的实验数据的分析表明,如果共溶质与洗涤剂的结合程度不高,则可以通过仅调整σ来解释不同的实验条件(例如水相中的共溶质)。该模型被认为是理论与实用性之间的良好折衷,可应用于膜蛋白的体外研究。

"点击查看英文标题和摘要"




































 京公网安备 11010802027423号
京公网安备 11010802027423号