Particuology ( IF 4.1 ) Pub Date : 2023-03-21 , DOI: 10.1016/j.partic.2023.03.004
Peiyi Yan , Ying Zhang , Shili Zheng
|
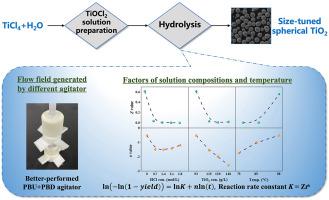
Hydrolysis of TiCl4 solution is capable of preparing microscale TiO2 particles. This research studied the synthesis of microscale spherical TiO2 powders and the hydrolysis kinetics. The effects of the flow field generated by different agitators and baffles in the crystallizer, the initial free acid concentration, the initial equivalent TiO2 concentration, and the temperature on the hydrolysis progress and powder morphology were systematically studied. The results show that the flow field in a crystallizer can significantly affect the morphology and particle size of the powders, and the axial flow can improve the sphericity of the powders. The increased free HCl and equivalent TiO2 concentrations in the pregnant solution inhibit the forward hydrolysis reaction, prolong the time to reach equilibrium, and reduce the yield. An appropriate temperature matching the compositions of the pregnant solution is crucial for the powder morphology and size. Powders with sizes ranging from around 5 μm–40 μm can be tuned under controlled flow field, solution compositions, and temperature conditions. In addition, the Cheng and Wunderlich modified Avrami equation was used for the crystallization kinetic modeling. The effects of the free HCl concentration, equivalent TiO2 concentration, and hydrolysis temperature are reflected in the reaction rate constant and active nuclei reduction index. Increasing the free HCl and equivalent TiO2 concentrations will reduce the reaction rate constant and accelerate the deactivation of the active nuclei, thus increasing the final powder size, while increasing the temperature will lead to the opposite results.
中文翻译:

通过 TiCl4 溶液水解制备的微型球形 TiO2 粉末:合成和动力学
TiCl 4溶液的水解能够制备微米级TiO 2颗粒。本研究研究了微型球形 TiO 2粉末的合成及其水解动力学。系统地研究了结晶器内不同搅拌器和挡板产生的流场、初始游离酸浓度、初始当量TiO 2浓度和温度对水解进程和粉末形貌的影响。结果表明,结晶器内的流场对粉体的形貌和粒径有显着影响,轴流可以改善粉体的球形度。增加的游离 HCl 和等效的 TiO 2母液中的浓度会抑制正向水解反应,延长达到平衡的时间,降低产率。与母液成分相匹配的适当温度对于粉末形态和尺寸至关重要。可以在受控的流场、溶液成分和温度条件下调整尺寸范围为 5 μm 至 40 μm 的粉末。此外,Cheng 和 Wunderlich 修改后的 Avrami 方程用于结晶动力学建模。游离HCl浓度、当量TiO 2浓度和水解温度的影响反映在反应速率常数和活性核减少指数中。增加游离 HCl 和等效的 TiO 2浓度会降低反应速率常数并加速活性核的失活,从而增加最终粉末的尺寸,而升高温度会导致相反的结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号